Keywords
Adalimumab, TNF-α, Psoriasis, CHAMPION, REVEAL, ADEPT, ESPRIT
Key points
- •
Adalimumab is a safe and effective therapy for the treatment of moderate-to-severe psoriasis.
- •
The mechanism of action of adalimumab is inhibition of tumor necrosis factor-α (TNF-α), thereby inhibiting the inflammatory cascade leading to psoriatic skin lesions.
- •
Pivotal trials on adalimumab therapy for the treatment of psoriasis and psoriatic arthritis include CHAMPION, REVEAL, and ADEPT.
- •
Adalimumab is generally safe and well-tolerated. Annual tuberculosis screening is recommended with adalimumab use.
Introduction
The advent of adalimumab therapy has greatly advanced the effectiveness of skin clearance in moderate-to-severe plaque psoriasis. Originally developed for the treatment of rheumatoid arthritis (RA) and psoriatic arthritis (PsA), adalimumab improves skin lesions in patients with psoriasis. Adalimumab was approved by the US Food and Drug Administration (FDA) for the treatment of moderate-to-severe chronic plaque psoriasis in 2008 and has since become a widely used therapy in the United States and abroad. It is also effective in erythrodermic and generalized pustular psoriasis and is additionally FDA approved for the treatment of RA, juvenile idiopathic arthritis, PsA, ankylosing spondylitis, adult Crohn disease, pediatric Crohn disease, ulcerative colitis, and hidradenitis suppurativa.
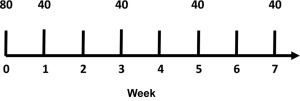
The current recommended dosing of adalimumab for psoriasis is subcutaneous (SC) injection of an 80-mg loading dose at baseline, 40 mg the following week, and maintenance on 40 mg every other week (EOW) thereafter ( Fig. 10.1 ). No definitive conclusions have been made regarding off-label omission of the loading dose. After a single dose, adalimumab levels peak in the bloodstream at approximately 5.5 days, with a half-life of approximately 2 weeks.
Available methods of SC injections include a pen and syringe, each containing 0.8 mL of 40 mg adalimumab. Self-injection is permitted so long as the dermatologist has carefully explained and evaluated the patient’s ability to do so. Injection sites should be alternated and should not be chosen in regions of bruised or tender skin. Patients tend to prefer the pen over the syringe, as it less painful, more convenient, faster, and safer.
Mechanism of action
Adalimumab is the first fully human recombinant immunoglobulin G1 monoclonal antibody that binds and neutralizes soluble and membrane-bound tumor necrosis factor (TNF), so that it cannot interact with p55 and p75 cell-surface TNF receptors. It also induces apoptosis in mononuclear cells with TNF receptors.
In regards to the pathogenesis of psoriasis and PsA, adalimumab inhibits specific events such as the release of serum cytokines (interleukin-6), acute phase reactants of inflammation, matrix metalloproteases, other markers of cartilage and synovium turnover, and the expression of adhesion molecules responsible for leukocyte migration. The inhibition of these events is thought to prevent epidermal cell hyperproliferation leading to psoriatic skin lesions.
Efficacy
Clinical trials on adalimumab therapy for psoriasis demonstrated remarkable results that led to its FDA approval. REVEAL and CHAMPION, phase 3 pivotal trials on the drug label, investigated the efficacy and safety profiles of adalimumab versus placebo (in controlled and interrupted therapy), and adalimumab versus placebo versus methotrexate (MTX), respectively ( Table 10.1 ).
| REVEAL | CHAMPION | ADEPT | |
|---|---|---|---|
| Eligibility criteria |
|
|
|
| Washout periods/exclusion criteria |
|
|
|
| Results |
|
|
|
REVEAL
REVEAL was a 52-week randomized, controlled trial that enrolled 1212 patients from 81 sites across North America. Adalimumab efficacy was assessed by 2 primary end points, achievement of Psoriasis Area Severity Index (PASI) -75 at week 16, and percentage of patients who lost an adequate response between weeks 33 and 52. The study population consisted of 53% of patients with moderate psoriasis, and 47% of patients with severe/very severe psoriasis by Physician’s Global Assessment (PGA).
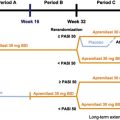
The study was divided into 3 periods: A, B, and C ( Fig. 10.2 ). During period A (weeks 0–15), patients were randomized 2:1 to receive standard recommended dosing of adalimumab or matched placebo dosing. Patients who achieved PASI-75 by week 16 were continued on to period B, whereas those who did not were eligible for a separate Open-Label Extension (OLE) study. During period B, the blind nature of the study was maintained by giving placebo-treated patients 2 injections of 40 mg adalimumab, and adalimumab-treated patients 2 injections of placebo. Then, all patients received 40 mg adalimumab EOW (weeks 16–33). Patients who achieved PASI-75 by week 33 were eligible to continue on to period C (weeks 34–52) to investigate whether adequate response would be lost with interrupted therapy (patients who achieved PASI-50 to PASI-75 response were eligible for the OLE study). Patients originally in the active therapy group from period A were rerandomized in a 1:1 ratio to receive adalimumab or placebo in order to assess for loss of response from weeks 33 to 52. Loss of response was defined as less than PASI-50 response after week 33 (relative to baseline score) and at least a 6-point increase in PASI score from week 33.
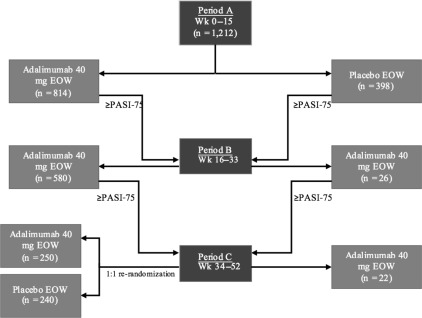
At week 16, 71% of adalimumab-treated patients from period A achieved PASI-75, compared with 7% of placebo-treated patients ( P <.001). Differences in PASI scores occurred by the first study visit at week 4, with mean PASI improvements of 52% versus 9% for placebo-treated patients ( P <.001). By week 16, 45% and 20% of adalimumab-treated patients had achieved PASI-90 and -100 scores, respectively, in comparison to 2% and 1% of placebo-treated patients ( P <.001).
The percentage of patients who lost adequate response after stopping adalimumab treatment was 28%, greater than the 5% who lost adequate response despite continuing adalimumab. In addition, loss of response occurred in a significantly shorter time period in patients stopping therapy.
REVEAL demonstrated rapid efficacy of adalimumab in psoriasis and that continuous treatment is more efficacious than interrupted treatment in maintaining adequate response ( Fig. 10.3 ).
Open-Label Extension Study from REVEAL
Patients from REVEAL and 3 other clinical trials were given the option to receive adalimumab therapy in a 3-year OLE trial. This trial aimed to determine additional long-term efficacy and safety data of continuous adalimumab therapy. By doing so, it surpassed the limitations of past trials, such as short duration of study (<1 year), the use of dosages not recommended by the FDA, and the exclusion of patients with less than a PASI-75 response.
Patients could enter this study in 1 of 4 groups: A through D. Group A consisted of patients with less than PASI-75 response at week 16 of REVEAL. Group B consisted of patients who had between PASI-50 and PASI-75 response at week 33 of REVEAL. Group C consisted of patients who received adalimumab during period C of REVEAL. Finally, group D consisted of patients who had initially received placebo during period A and then received adalimumab in period B of the OLE study. Patients with less than PASI-50 response at week 33 and patients who were rerandomized to receive placebo in period C of REVEAL were excluded from this study.
The patients in groups A–D were scheduled to receive uninterrupted treatment with 40 mg SC adalimumab EOW for a minimum of 108 weeks, or until dose escalation. Dose escalation to 40 mg weekly was permitted for patients who did not achieve PASI-50 by week 24 of the OLE study. During this time, patients were permitted to continue shampoos, emollients, and corticosteroids on the palms/soles/inframammary areas, and groin, as long as it was not within 24 hours of a study visit. Phototherapy and other systemic therapies were not permitted.
Adalimumab efficacy was well maintained for up to 3 years of continuous therapy in those with sustained PASI-75 responses in the first 33 weeks of therapy ( Table 10.2 ). Efficacy was best maintained in those who achieved PASI-100.
| Group | PASI-75 Rate | Additional Efficacy Information |
|---|---|---|
| A | 79% (week 160) | 40% mean percentage improvement after 3 y of continuous therapy |
| B | 70% (week 165) | 44% mean percentage improvement after 3 y of continuous therapy |
| C | 76% (week 160) | 93% mean percentage improvement after 33 wk of therapy PASI-75/90/100 response rates after 160 wk was 76%/50%/31% |
| D | 89% (week 160) | 73% mean percentage improvement after 24 wk of therapy PASI-75 response rates at weeks 64/100/160 were 78%/88%/89% |
Comparative Study of Adalimumab Versus Methotrexate Versus Placebo in Patients with Psoriasis
Efficacy and safety results from the randomized, controlled comparative study of adalimumab versus MTX versus placebo in patients with psoriasis (CHAMPION) demonstrated the superiority of adalimumab to placebo, and the noninferiority of adalimumab to MTX, a widely used systemic treatment.
The study enrolled 271 patients from 28 different sites across Europe and Canada and randomized them in a 2:2:1 ratio to receive either adalimumab, MTX, or placebo for 16 weeks. Adalimumab dosing was SC injection of 80 mg at week 0, and 40 mg EOW. Oral MTX was dosed at 7.5 mg at week 0, increased to 10 mg/wk at week 2, and finally, to 15 mg/wk at week 4. Patients who achieved PASI-50 by week 8 were maintained on the 15 mg/wk dose, and those who did not were increased to 20 mg/wk. Any patient not achieving PASI-50 by week 12 was increased to a maximum study dose of 25 mg/wk. The primary method of assessing efficacy between the 2 agents and placebo was by the proportion of patients that achieved PASI-75 in 16 weeks.
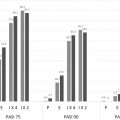
The baseline mean PASI score for the patients in this study was 19.7, and mean body surface area (BSA) coverage was 32.1%. At 16 weeks, 79.6% of patients receiving adalimumab achieved PASI-75 versus 35.5% of patients receiving MTX and 18.9% of patients receiving placebo ( Fig. 10.4 ). PASI-100 was achieved by 16.7% of adalimumab patients, 7.3% of MTX patients, and 1.9% of placebo patients. Response to adalimumab therapy was rapid, indicated by a 57% improvement in PASI score by week 4 of follow-up.
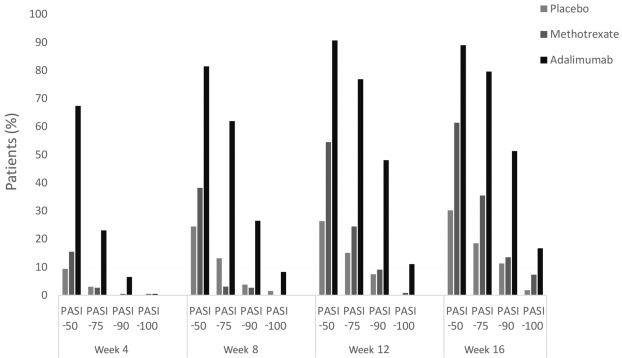
Adalimumab was more effective than MTX and placebo and rapidly improved symptoms. The CHAMPION trial was the first time a biologic agent was compared head to head with MTX for psoriasis.
Efficacy in Psoriatic Arthritis
The ADEPT trial was a pivotal, double-blinded, randomized, placebo-controlled trial designed to evaluate safety and efficacy of adalimumab therapy for moderate-to-severe PsA.
The 315 study patients were divided based on history of MTX use and degree of psoriasis (<3% or ≥3% BSA involvement) and randomized in a 1:1 ratio to receive SC injections of either placebo or 40 mg adalimumab EOW. Primary efficacy endpoints were American College of Rheumatology (ACR) 20 response score at week 12 and any change in the modified total Sharp score of radiographic structural damage of the hands and feet at week 24 from baseline. An important secondary efficacy end point was the improvement of cutaneous psoriasis as assessed by achievement of PASI-50 and PASI-75.
At week 12, ACR20 was achieved by 58% in the adalimumab group and 14% in the placebo group ( Fig. 10.5 ). ACR20, -50, and -70 responses did not differ between patients taking adalimumab in combination with MTX and patients taking adalimumab alone. Radiographs of patients receiving adalimumab indicated inhibition of structural changes as well, with a change in modified total sharp score of −0.2 for adalimumab patients and 1.0 for placebo patients at week 24.
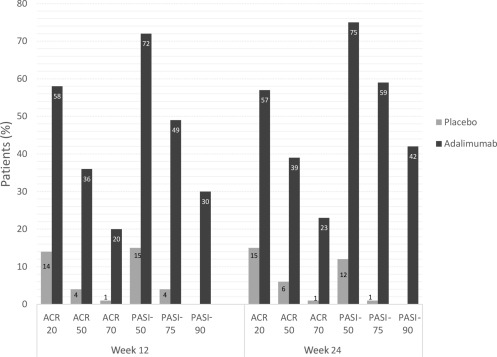
The initial degree of psoriasis was similar between the placebo and adalimumab groups. PASI-75 was achieved by 59% for adalimumab and 1% of placebo patients at week 24 (n = 69).
This trial demonstrated significant improvement in the joint and skin manifestations of PsA and cutaneous psoriasis, resulting in the breakthrough of adalimumab as a potential first-line therapy for these conditions.
Expert Recommendations on Adalimumab Efficacy
Clearance of skin lesions is better maintained with uninterrupted administration of adalimumab injections, although rebound does not commonly occur upon discontinuation of therapy. There is, however, loss of efficacy after the restart of adalimumab therapy. Studies investigating dose escalation from 40 mg EOW to 40 mg every week showed that this may only be beneficial in a small fraction of patients, specifically those who lost response to EOW dosing.
Safety
Adalimumab is a well-tolerated drug with minimal side effects in most patients. Given the role of TNF in host defense, the most common concern with adalimumab is the risk of infection and malignancy. Serious infections include the reactivation of tuberculosis (TB), bacterial sepsis, invasive fungal infections, and infections due to opportunistic pathogens. Concerns for malignancy include lymphoma and nonmelanoma skin cancer (NMSC).
The most common adverse reactions in placebo-controlled clinical trials with adalimumab were injection site reaction, seen in 20% of patients receiving active therapy versus 14% of patients receiving placebo. These reactions (erythema, itching, hemorrhage, pain, or swelling) were generally mild-to-moderate and did not require discontinuation of the drug. Other common adverse reactions include mild-to-moderate infection, such as upper respiratory infection, or sinusitis, headache, or rash. Adalimumab is pregnancy category B.
Risk of Infection and Malignancy
Multiple studies have examined the risks of infection and malignancy associated with the use of biologic agents. The PSOLAR (Psoriasis Longitudinal Assessment and Registry) registry was designed to detect adverse events (AEs) over a 6-year period in psoriasis patients using various therapies. The registry enrolled patients on various treatments; the patients were not randomized to the different treatments. Serious infection rates were higher with adalimumab and infliximab in comparison to non-MTX and nonbiologic therapies; the risk of serious infections associated with ustekinumab and etanercept was not increased compared with non-MTX and nonbiologic treatment.
The SABER (Safety Assessment of Biologic Therapy) study, which combined data on patients with autoimmune disease (RA, inflammatory bowel disease, psoriasis, PsA, and ankylosing spondylitis) from 4 large US databases, found no increased risk with TNF inhibitor therapy as a group for hospitalization for serious infections, compared with initiation of nonbiologic medications. The study also examined the incidence of cancer following TNF inhibitor therapy and found no increase in the incidence of solid cancer compared with disease-specific alternative therapy for all immune-mediated diseases. For diseases and cancer types wherein there was a sufficient number of events to estimate risk, no significantly increased risk was detected for lymphoma, leukemia, or NMSC.
ESPRIT 5-year Analysis
The ESPRIT surveillance registry is an ongoing, 10-year observational after-marketing registry designed to prospectively evaluate the long-term safety and efficacy of adalimumab therapy. Five-year results were recently published in July 2015, showing no new safety signals from previous trials.
Eligibility criteria included patients 18 years of age or older with a diagnosis of chronic plaque psoriasis, who initiated adalimumab within 4 weeks of registry entry, initiated adalimumab without being off the drug for more than 70 consecutive days, or participated in a “feeder trial” (adalimumab clinical trial) without being off the drug for more than 70 days after completion of the study. The registry consisted of 2 populations: the “all-treated population” (received at least 1 dose of adalimumab during the registry) and the “new prescription” population (all-treated population patients that received first dose within 4 weeks of registry entry). Patients could continue on concomitant psoriasis therapy with the exception of anakinra, abatacept, or another biologic agent.
Patients were exposed to adalimumab for 13,639 patient-years (PY) during the registry and 19,242 years total ( Table 10.3 ). Over the course of the 5 years, discontinuation rates were 10.6% and 13.1% for the all-treated and new prescription populations, respectively, with the most common reason for discontinuation being loss to follow-up. The rate of serious treatment- emergent adverse events (TEAE) was 4.3/100 PY of total adalimumab exposure, with the most serious TEAE being infection (1/100 PY). Myocardial infarction (MI) was the most common event leading to death (<0.1 Event/100 PY).