18 Acute management of burn/electrical injuries
Synopsis
 Learn assessment and classification of burn wounds, including estimation of burn size and depth, and reduction of related morbidity and mortality.
Learn assessment and classification of burn wounds, including estimation of burn size and depth, and reduction of related morbidity and mortality.
 Gain an appreciation of stress response to acute burn injury, including hemodynamic, metabolic, nutritional and immunologic sequelae.
Gain an appreciation of stress response to acute burn injury, including hemodynamic, metabolic, nutritional and immunologic sequelae.
 Learn initial management of the acute burn patients, including fluid resuscitation, nutritional support and wound care.
Learn initial management of the acute burn patients, including fluid resuscitation, nutritional support and wound care.
 Learn wound management of burn patients including an understanding of wound healing, wound sepsis, topical antimicrobial agents, biological dressings, skin substitutes and skin grafts.
Learn wound management of burn patients including an understanding of wound healing, wound sepsis, topical antimicrobial agents, biological dressings, skin substitutes and skin grafts.
 Learn fundamental surgical principles in treatment of burn patients, including wound debridement, wound dressing and splinting, skin grafting and scar contracture release.
Learn fundamental surgical principles in treatment of burn patients, including wound debridement, wound dressing and splinting, skin grafting and scar contracture release.
 Burn rehabilitation, including physical/occupational therapy, psychosocial support and reconstructive needs.
Burn rehabilitation, including physical/occupational therapy, psychosocial support and reconstructive needs.
 Principles of management of special problems, including inhalation injuries, chemical burns, electrical injuries and toxic epidermal necrolysis.
Principles of management of special problems, including inhalation injuries, chemical burns, electrical injuries and toxic epidermal necrolysis.
Acute management of burn injuries
Rescue: The aim is to get the individual away from the source of the injury and provide first aid.
Resuscitate: Immediate support must be provided for any failing organ system. This usually involves administering fluid to maintain the circulatory system but may also involve supporting the cardiac, renal, and respiratory systems.
Retrieve: After initial evacuation to an accident and emergency department, patients with serious burns may need transfer to a specialist burns unit for further care.
Resurface: The skin and tissues that have been damaged by the burn must be repaired. This can be achieved by various means, from simple dressings to aggressive surgical debridement and skin grafting.
Rehabilitate: This begins on the day a patient enters hospital and continues for years after he or she has left. The aim is to return patients, as far as is possible, to their pre-injury level of physical, emotional, and psychological wellbeing.
Reconstruct: The scarring that results from burns often leads to functional impairment that must be addressed. The operations needed to do this are often complex and may need repeating as a patient grows or the scars re-form.
Review: Burn patients, especially children, require regular review for many years so that problems can be identified early and solutions provided.
Epidemiology
The incidence of burn injuries varies from country to country, typically peaking during the country’s holiday period. According to the most recent statistics compiled by the World Health Organization and the World Fire Statistics Center, fires caused 6.6 million major burn injuries and 400 000 deaths every year.1 The burden of burn injury falls predominantly on the world’s poor; 95% of fire-related burns occur in low- and middle-income countries where prevention programs are almost nonexistent and open fires for cooking, lighting or heating are commonplace. The average death rates are about three fire deaths per 100 000 inhabitants and one fire death per 100 fires. However, this average conceals a more than 100-fold variation in death rates from country to country. A better indication of typical fire risk is the median fire death rate per 100 000 inhabitants by country, which was 0.9 in 2004. In addition, the WFSC released fire-related death data by country (from lowest to highest number of deaths per 100 000 person) from 2002–2004. The countries with the lowest incidences include Singapore (0.08) and Switzerland (0.51). Those with the highest include Finland (2.08) and Hungary (2.10).The cost to society in terms of lost wages, vocational rehabilitation, and need for long-term care is staggering. Worldwide, severe burns cause disabilities that cost $80.2 billion a year in lost productivity (wages and skills) alone; medical expenses would add millions more. Lost productivity costs the world billions of dollars annually. In 2009, the WFSC noted that the cost of direct fire losses ranged from 0.06–0.26% of countries’ gross domestic product (GDP) and the cost of indirect fire losses ranged from 0.002–0.95% of countries’ GDP.
Mechanisms of thermal injury
Types of burns
Thermal burns
Scalds
Hot water scalds are the most common cause of pediatric burn admissions. They also often occur in elderly people. The common mechanisms are spilling hot drinks or liquids or being exposed to hot bath water (Fig. 18.1). The depth of scald injury depends on the water temperature, the skin thickness and the duration of contact. Water at 60°C creates a deep dermal burn in 3 s but will cause the same injury in 1 s at 69°C. Boiling water often causes a deep dermal burn, unless the duration of contact is very short. Grease and hot oils will generally cause deep dermal or deeper burns.
Contact burns
In order to get a burn from direct contact, the object touched must either have been extremely hot or the contact abnormally long. The latter is a more common reason, and these types of burns are commonly seen in people with epilepsy or those who misuse alcohol or drugs. They are also seen in elderly people after a loss of consciousness; such a presentation requires a full investigation as to the cause of the blackout (Fig. 18.2). Contact burns commonly result from hot metals, plastics, glass or hot coals. Burns from brief contact with very hot substances are usually due to industrial accidents. Although generally small in size, contact burns are challenging in that the injury tends to be deep dermal or full thickness.
Tar
Numerous substances have been used in the past with variable results and selection of the appropriate agent for the removal of adherent tar is still challenging. Polyoxyethylene sorbitan, an emulsifying agent commonly used as a base in ointments, separates the tar from the skin and, as it is water soluble, easily washes off.2
Alternatively silver sulfadiazine, neosporin ointment (with polyoxyethylene sorbitan as a base), polysorbate or De-Solv-It (a citrus petroleum distillate with surfactant and lanolin) may be left on the burn, which is then bandaged. The tar comes away with the bandages when they are removed the next day. These may be used by themselves or in combination with an antibiotic ointment.3 With some of these agents, it is recommended to leave it on for 12–48 h at a time until the tar has dissolved.
Mechanical or manual debridement is painful, relatively ineffective, and results in the removal of underlying viable skin and hair follicles, thus extending the depth and area of the dermal injury. In addition, a degree of autodebridement will occur. Debriding is a balance between removing the tar and exposing the injured skin for evaluation and treatment. Judgment should be exercised as to how much debridement is appropriate in the emergency setting, as extensive debridement may require moderate-to-deep sedation. If the skin has a light coat of tar and the patient does not complain about the underlying skin or surrounding tissue, leaving the asymptomatic tar in place may be acceptable.4 It has been reported that early excision and grafting may be required in some patients and this will decrease the hospitalization times. Tar that is part of an obvious burn, blister, or tissue loss should be removed and conjunctival tar, should be removed by an ophthalmologist.
Chemical burns
See ‘Cold and chemical injury to the upper extremity,’ Chapter 20 for details of chemical burns.
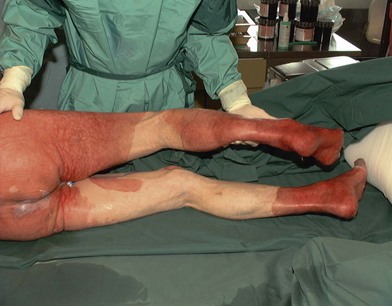
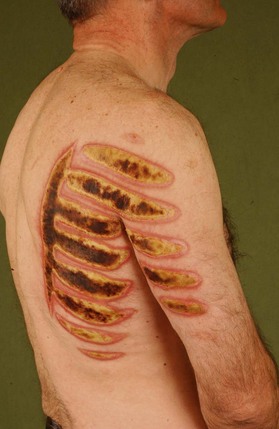
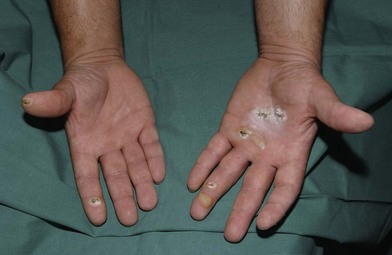
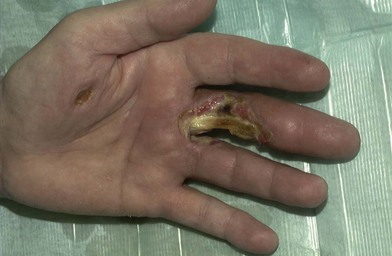
Electrical burns
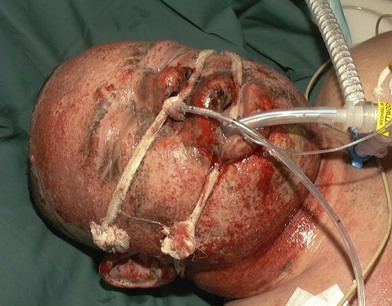
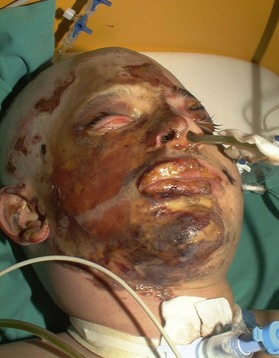
Thermal injury due to electrical current is defined as tissue injury by exposure to supraphysiological electrical currents (Table 18.1). Electrical burns (Figs 18.3, 18.4) are classified as high voltage (≥1000 V), low voltage (<1000 V), ‘flash burn’ (in which there is no electrical current flow through the body of the patient) and burns caused by lightning.
Table 18.1 Physiologic effects of different electrical currents
| Effect current (milliamps) | |
|---|---|
| Tingling sensation/perception | 1–4 |
| Let-go current | 3–9 |
| Skeletal muscle tetany | 16–20 |
| Respiratory muscle paralysis | 20–50 |
| Ventricular fibrillation | 50–120 |
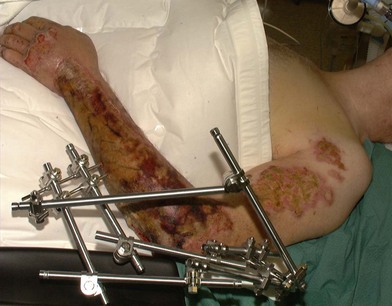
Aggressive treatment including surgical debridement of devitalized tissues, or those with doubtful viability, frequently exposes vital structures in patients who have suffered high-voltage burns. The problem of ‘high risk’ wounds warranting priority in providing an early and emergency cover compounded with paucity and limitations of choice of procedures throw a great challenge to the surgeon. These require a higher necessity of flaps when compared with other burn groups, due to the characteristic compromising of deeper structures. High complication rates are reported with electrical injury including partial or complete flap failure. This fact, associated with the need to preserve the vital organs and structures like vessels, bones, tendons and nerves justifies the aforementioned cautious reconstructive approach with serial examination and debridement prior to a definitive surgery. The vascular damage and resulting thrombotic phenomenon abates during second week and tissues become healthy. This is the time when tissues around the wound withstand manipulation for a local, regional, axial or free flap. The mortality rate of patients who suffer high-voltage burns ranges from 0 to 21.7%; their main cause of death is multiorgan failure secondary to sepsis of cutaneous origin.5,6
Myonecrosis, with severe myoglobinemia as a result of a massive muscular destruction can lead to acute renal failure, despite aggressive volemic replacement. As the extent of injury cannot be quantified as in a cutaneous burn, fluid resuscitation must be adjusted to urine output. The treatment of myoglobinuria includes initial stabilization and resuscitation of the patient while concomitantly attempting to preserve renal function. Early aggressive fluid replacement is beneficial in minimizing the occurrence of renal failure. While osmotic diuretics (e.g., mannitol) and alkalinizing agents (e.g., bicarbonate) are considered the standard of care in preventing acute renal failure in patients with myoglobinuria, there is little clinical evidence to support the use of these agents.7
Pathophysiology
Mediators of burn injury
Hints and tips
• A burn results in three distinct zones: coagulation, stasis, and hyperemia
• The aim of burns resuscitation is to maintain perfusion of the zone of stasis
• Systemic response occurs once a burn is greater than 30% of total body surface area
• Different burn mechanisms lead to different injury patterns.
Initial evaluation and treatment
Treatment of a burned patient starts at the scene of injury with the safe removal of the patient from the cause of the burn. In all cases (especially if chemical or electrical burns), care must be taken to avoid personal injury by checking the area is safe and that appropriate protective clothing is worn if necessary. Priority should be given to assessing the person’s airway, breathing, and circulation, and presence of any coexisting injuries which may require more urgent treatment than the burn. Belts, clothes, jewelry and watches that can retain heat and cause constriction should be removed and the patient kept aware. An inhalation injury should be assumed and airway should checked and oxygen given. Heat can be dissipated from the burn wound by cooling with water but cold water and ice should not be used as they can cause rapid hypothermia. A quick assessment should be made for associated trauma and the patient transported to the nearest burn unit for definitive management (Box 18.1, Fig. 18.5).
Box 18.1
Burn injuries that should be referred to a burn unit
1. Partial thickness burns >10% TBSA and patients requiring burn shock resuscitation
2. Burns that involve the face, hands, feet, genitalia, perineum, or major joints
3. Deep partial thickness burns and full thickness burns in any age group
4. Circumferential burns in any age group
5. Electrical burns, including lightning injury
7. Burns with a suspicion of inhalation injury
8. Burns of any size with concomitant trauma or diseases which might complicate treatment, or prolong recovery, or affect mortality
9. Diseases associated to burns such as toxic epidermal necrolysis, necrotizing fasciitis, staphylococcal scalded child syndrome etc., if the involved skin area is 10% for children and elderly and 15% for adults or any doubt of treatment
10. Any patients with burns and concomitant trauma (such as fractures) in which the burn injury poses the greatest risk of morbidity or mortality. In such cases, if the trauma poses the greater immediate risk, the patient may be initially stabilized in a trauma center before being transferred to a burn unit. Physician judgment will be necessary in such situations and should be in concert with the regional medical control plan and triage protocols
11. Any type of burns if any doubt about the treatment
12. Burned children in hospitals without qualified personnel or equipment for the care of children
13. Burn injury in patients who will require special social, emotional, or long-term rehabilitative intervention
Modified from: European Burns Association. European Practice Guidelines for Burn Care & Guidelines for the Operations of Burn Units; 2002:55–62; Resources for Optimal Care of the Injured Patient; 1999; Committee on Trauma, American College of Surgeons; 1999.
Once the patient arrives at the emergency room, an evaluation of the Airway, Breathing, and Circulation (the ABCs) should receive first priority (Box 18.2). It is also important to exclude associated trauma. The history should include the time, location and circumstances of the injury, where the patient was found, and their condition (Box 18.3). Past medical and social history, current medication usage, drug allergies, and tetanus status should be rapidly determined. It is also important to consider the possibility of nonaccidental burns or scalding (Box 18.4).
Box 18.2
Initial assessment of a major burn
Perform an ABCDEF primary survey:
A – Airway with cervical spine control
E – Exposure with environmental control
• Establish good intravenous access and give fluids (in children, the interosseous route can be used for fluid administration if intravenous access cannot be obtained)
• Catheterize patient or establish fluid balance monitoring
• Take baseline blood samples for investigation (full blood count; urea and electrolyte concentration; clotting screen; blood group; and save or cross-match serum)
• After completion of the primary survey, a secondary survey should assess the depth and TBSA burned, reassess, and exclude or treat associated injuries
Box 18.3
Key points of a burn history
Inhalational injuries
• Is there risk of inhalational injuries (did burn occur in an enclosed space)?
• If possible, the nature of burning materials (furniture, polyurethane foam, polyvinyl chloride, etc.)
Box 18.4
Indicators of possible nonaccidental burns or scalds
• Historical accounts of injury differ over time
• History inconsistent with the injury presented or with the developmental capacity of a child
• Past abuse or family violence
• Inappropriate behavior/interaction of child or caregivers
• Glove and sock pattern scalds
• Scalds with clear-cut immersion lines
• Absence of splash marks in a scald injury. A child falling into a bath will splash; one that is placed into it may not
• Burns to palms, soles, genitalia, buttocks, perineum
• Is there sparing of flexion creases – that is, was child in fetal position (position of protection) when burnt? Does this correlate to a “tideline” of scald – that is, if child is put into a fetal position, do the burns line up?
• “Doughnut sign,” an area of spared skin surrounded by scald. If a child is forcibly held down in a bath of hot water, the part in contact with the bottom of the bath will not burn, but the tissue around will
• Symmetrical burns of uniform depth
• Restraint injuries on upper limbs
• Other signs of physical abuse – bruises of varied age, poorly kempt, lack of compliance with healthcare (such as no immunizations)
A thorough assessment of a person with a burn should then take into account:
• The type of burn (e.g., flame, scald, electrical, or chemical)
• The depth and extent of the burn, and therefore the severity
• The risk of inhalation injury (singed nasal hair, black carbon in the sputum, or carbon in the oropharynx) (Box 18.5)
• Any coexisting medical conditions (e.g., cardiac, respiratory, or hepatic disease; diabetes; pregnancy; or immunocompromised state)
• Any predisposing factors which may require further investigation or treatment (e.g., a burn resulting from a fit or faint)
• The possibility of nonaccidental injury
• The person’s social circumstances (e.g., ability to self-care or need for admission).
The airway should be secured because upper airway obstruction can develop quickly (Fig. 18.6). Smoke inhalation causes more than 50% of fire-related deaths. Patients sustaining an inhalation injury may require aggressive airway intervention (Fig. 18.7). Most injuries result from the inhalation of toxic smoke; however, super-heated air may rarely cause direct thermal injury to the upper respiratory tract (see complications, below). Patients who are breathing spontaneously and at risk for inhalation injury should be placed on high-flow humidified oxygen. Patients trapped in buildings or those caught in an explosion are at higher risk for inhalation injury. These patients may have facial burns, singeing of the eyebrows and nasal hair, pharyngeal burns, carbonaceous sputum, or impaired mentation. A progressive change in voice quality or hoarseness, stridorous respirations, or wheezing may be noted. The upper airway may be visualized by laryngoscopy, and the tracheobronchial tree should be evaluated by bronchoscopy. Chest radiography is not sensitive for detecting inhalation injury. Patients who have suffered an inhalation injury are also at risk for carbon monoxide poisoning. The pulse oximeter is not accurate in patients with carbon monoxide poisoning because only oxyhemoglobin and deoxyhemoglobin are detected. Co-oximetry measurements are necessary to confirm the diagnosis of carbon monoxide poisoning. Other pulmonary assessments include arterial blood gas measurements and bronchoscopy. Patients exposed to carbon monoxide should receive 100% oxygen using a nonrebreather facemask.
Burn assessment
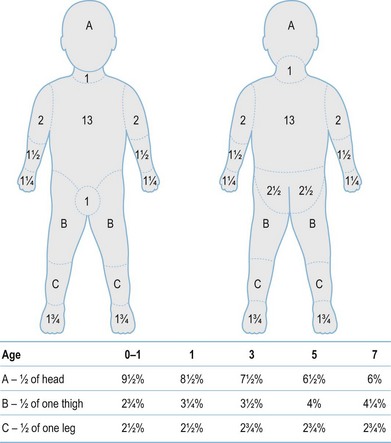
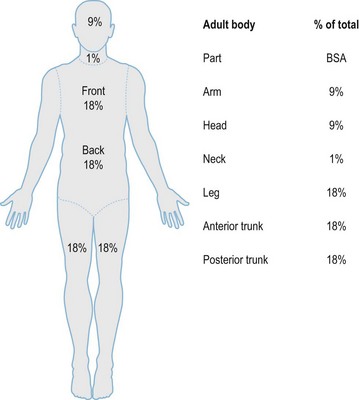
After completion of the primary survey, a secondary survey must include assessment of the depth and TBSA burned (Figs 18.8–18.10).
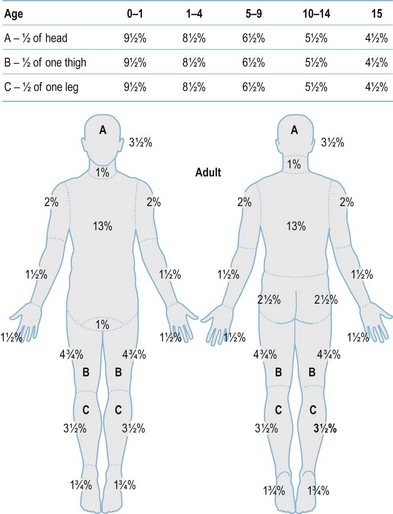
Fig. 18.8 Estimation of burn size with the Lund and Browner method.
(From: Herndon DN, ed. Total Burn Care, 2nd edn. Edinburgh: Elsevier.)
Diagnosis/patient presentation
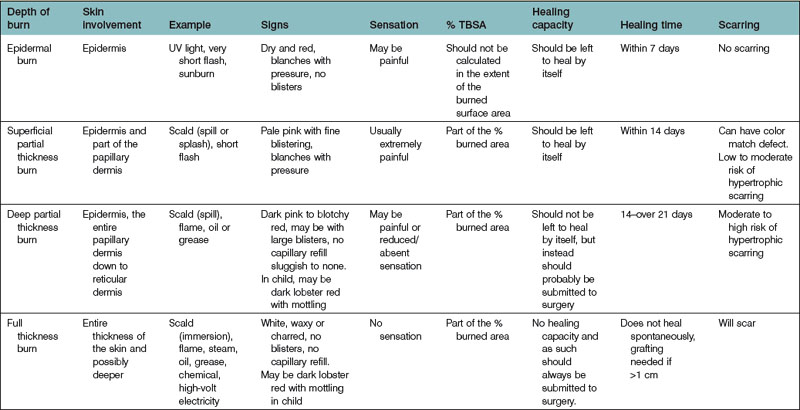
Accurate assessment of burn depth on admission is important in making decisions about dressings and surgery (Table 18.2). However, the burn wound is a dynamic living environment that will alter depending on both intrinsic factors (such as release of inflammatory mediators, bacterial proliferation) and extrinsic factors (such as dehydration, systemic hypotension, cooling). There is much evidence to demonstrate the beneficial effects of cooling on reducing tissue damage and wound healing time. Although immediate cooling is preferable, even a 30 min delay in application of cooling is still beneficial to the burn wound but the application of cooling 60 min after injury, does not demonstrate any benefit.8 Tetanus prophylaxis must be considered wherever there is tissue damage, and particularly in elderly patients. It is therefore important to review the wound at regular intervals until healing.
Optimum treatment of the wound reduces morbidity and, in larger injuries, mortality. It also shortens the time for healing and return to normal function and reduces the need for secondary reconstruction (Box 18.6). When epithelialization is delayed beyond 3 weeks, the incidence of hypertrophic scarring rises. Hypertrophic scars occur in 60% of burned children aged under 5 years. Optimal burn care requires early excision and grafting of all burns that will produce hypertrophic scars (typically those that will not or have not healed within 3 weeks of the injury), so an accurate estimation of burn depth is crucial. Early grafting of those burns that have not healed at three weeks has been shown to improve the result, but because of delays in the referral process, all injuries, which show no sign of healing by 10 days, should be referred for assessment. The appearance of the wound – and the apparent burn depth – changes dramatically within the first 7–10 days. A burn appearing shallow on day 1 may appear considerably deeper by day 3. This demarcation of the burn is a consequence of thrombosis of dermal blood vessels and the death of thermally injured skin cells. Superficial burns may convert to deeper burns due to infection, desiccation of the wound, or the use of vasoactive agents during resuscitation from shock.