Fig. 11.1
Elastosis perforans serpiginosa. Conditions such as elastosis perforans serpiginosa (EPS) may be confused with acquired perforating dermatosis (APD). However, the annular distribution seen here is common in EPS, yet rare in APD. Courtesy of Julia R. Nunley, M.D.
Characteristic clinical findings, proposed by Faver et al. to be seen as diagnostic criteria are: hyperkeratotic or cup-shaped centrally depressed papules, transepidermal elimination of basophilic collagen bundles, manifestation in adults over 18 years of age [5, 6].
APD typically starts as small papules of 1 mm which develop in a few weeks into umbilicated papules and plaques with firmly adherent “crusts” measuring up to 0.5–2.0 cm in diameter; lesions may occasionally coalesce to form plaques of several centimeters. The border is erythematous and slightly elevated, as a ridge. The color of the crust varies from light to black to brown-green, sometimes with an oyster-shell appearance. The number of lesions ranges from just a few to many. Lesions typically are grouped, solitary disseminated papules are less common; some lesions form as a manifestation of the Köbner phenomenon. Forceful removal of the plug will result in a bleeding crater. Elevated, plateau-like, or depressed lesions occur. After detachment of the crust, a crater-like lesion remains, healing by 2 months leaving an atrophic, depressed, hypopigmented or hyperpigmented scar. Although each individual lesion has a limited evolution time, the eruption in one individual may last many years (Fig. 11.2).
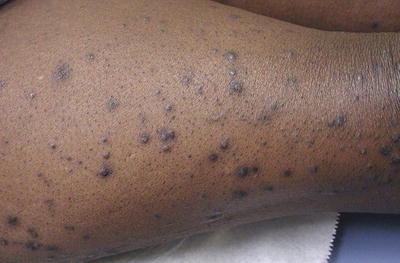

Fig. 11.2
Seen here are typical cone-shaped hyperpigmented, hyperkeratotic papules of acquired perforating dermatosis in a diabetic on hemodialysis. Courtesy of Julia R. Nunley, M.D.
Sites commonly affected include the extensor aspects of the extremities, the trunk, shoulder girdle, the gluteal region, and sometimes the head. Mucous membranes, folds or intertriginous areas, and palmoplantar regions are spared.
Pruritus is the presenting symptom in about three-fourths of patients, although pruritus is not universal; a minority of patients are asymptomatic and others report pain. Some with pruritus report sporadic bouts of severe, unrelenting itch.
Pathogenesis, Etiology
The pathogenesis is unclear but several hypotheses have been developed to explain the phenomenon.
Suggested causes include: a genetic predisposition (to be distinguished from the inherited forms), associated diseases, and occasionally certain medications [7].
The main abnormality in the primary reactive perforating disorders is a focal damage to or alteration of the extracellular matrix collagen fibers, or deposition of material foreign to normal dermis, and a subsequent elimination of this altered material through the epidermis.
There is indirect evidence that mild superficial trauma plays a role: the frequent association with pruritus and scratching; development of lesions on reachable sites and superficial trauma-prone areas; the observed Köbner phenomenon; and the lack of lesion development following deeper wounds such as surgical incisions.Alterations of extracellular matrix-components may be secondary to inflammation, metabolic alteration, neoplastic cells, or deposition of aberrant external substances. These papillary dermal changes induce an active epidermal response consisting of downward proliferation to engulf and isolate the material, thereby creating perforating transepithelial channels to extrude the altered components. The proliferative epidermis is sometimes seen as pseudoepitheliomatous hyperplasia surrounding the central plug.
Mehregan identifies this as an active process, distiguishable from the more “passive” transmigration and desquamation through epidermal turnover experienced by small particles such as hemosiderin, or the active migration and epidermal elimination of motile cells such as neutrophils or infectious such as Treponema pallidum [6]. Following the elimination of material in these cases, the epidermis will return to normal, although sometimes leaving a scar.
Through which signal transepidermal elimination is induced is a matter of debate. Exogenous foreign material, deposition of urate crystals or calcium hydroxyapatite, both infectious and non-infectious granulomas, altered dermal components all are stimuli for the epidermis to respond with downward proliferation, engulfing and elimination of these altered substances. Close proximity to the dermal–epidermal interaction zone seems to be a condition sine qua non: according to experimental studies the critical level would be that of the hair papillae; deep granulomas, deeper trauma, or foreign material will not induce the response [8].
In APD associated with CKD, deposition of substances that cannot be removed with dialysis is a proposed theory. Matrix and epidermal changes related to metabolic diseases have been suggested to play a role. Hyaline degeneration of collagen fibers in advanced diabetes may be due to glycation and lipoxidation of proteins and other compounds, resulting in advanced glycation end products (AGEs). The resulting Mailliard adducts remain in proteins with a long half-life and change their physical/biochemical properties. The induced molecular cross-linking impairs the solubility, susceptibility, and reaction ability of these altered structural biological proteins and fibers. In patients with chronic renal insufficiency, the elimination of these Maillard products is impaired. One hypothesis is that microtrauma to the skin may trigger the elimination of such altered collagen fibers.
Diabetic microangiopathy is considered to be a predisposing factor facilitating necrosis or necrobiosis of metabolically altered subepidermal components by minor trauma.
Histopathology
Characteristic feature of the perforating dermatoses is the transepidermal elimination of various substances such as keratin, collagen, and elastic fibers. Histological features of APD are not uniform; they may resemble any of the four perforating disorders mentioned above. Histopathological examination will vary according to evolution and clinical subtype, resembling specific perforating disorders (PF, APD, KD, EPS).
Histologic findings are relative to the evolutionary stage of the lesion.
Early lesions show complete disappearance of the basal lamina and although desmosomes are intact, bundles of collagen fibers appear in widened intercellular spaces. In excoriated lesions (due to scratching) eosinophilic necrotic material may be seen containing pycnotic nuclei of inflammatory cells and degenerated collagen bundles lying in continuity with those in the dermis. The epidermis in these excoriated lesions may show any stage of regenerative healing.
The fully develop umbilicated papule shows a typical cup-shaped interruption in the epidermis containing cellular debris and basophilic collagen bundles in its center. Content includes cellular debris, neutrophilic granulocytes, and sometimes bacteria. The degenerated collagen fibers are oriented vertically, perpendicular to the skin surface which is parakeratotic in the central zone of this plug, in what appears to be narrow tunnels of elimination.
The lateral borders are sharply delineated with epithelial hyperplasia, sometimes with hypergranulosis and hyperkeratosis. There is often a mild superficial inflammatory infiltrate of mixed cells, with lymphocytes, neutrophils, and histiocytes.
In resolving lesions the crater is shallow, containing remnants of degenerated collagen and parakeratotic keratin, while the base of the plug consists of almost regenerated epidermis, with a partially organized basement membrane. A repair phenomenon will reconstitute the epidermis and close the eliminating channels. Along with epidermal turnover the hyperkeratotic plug gradually will slough off.
The clinical and histopathological subtype of APD is determined by the content of the eliminated material.
The lesions resembling KD show epidermal invagination filled with a keratotic plug containing cellular debris and neutrophils, but no collagen nor elastin. In those presenting like RPC, the cup-shaped invagination of the epidermis is filled with a plug containing keratin, cellular debris and neutrophils; vertically oriented collagen bundles can be seen at the base of the cup, which are eliminated in a transepidermal way. The refractile hyalinized collagen fiber bundles in the base of the plug stain basophilic with hematoxylin–eosin and red with Van Gieson staining.
Lesions resembling PF show a dilated infundibulum filled with necrotic debris, orthokeratotic and parakeratotic keratin, and inflammatory cell debris. This follicular content is exposed to the dermis, and the resulting inflammatory reaction is a perifollicular mononuclear of mixed type of inflammatory infiltration believed to lead to transepidermal elimination.
In APD associated with CKD, only rarely will elimination of degenerated elastic fibers and cellular debris be seen as a cup-shaped epidermal invagination; this is more characteristic of EPS.However, when present, histochemical staining reveals an increase in coarse and degenerative dermal elastic fibers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








