12 Abdominal wall reconstruction
Synopsis
 Abdominal wall reconstruction techniques are indicated for hernia repair, reconstruction of tumor defects, congenital defects, and correction of traumatic defects (e.g., from damage control laparotomies).
Abdominal wall reconstruction techniques are indicated for hernia repair, reconstruction of tumor defects, congenital defects, and correction of traumatic defects (e.g., from damage control laparotomies).
 Patients may be complicated by fistulae, adhesions, infections, scarring from previous injury or surgery, and presence of dehisced prior mesh.
Patients may be complicated by fistulae, adhesions, infections, scarring from previous injury or surgery, and presence of dehisced prior mesh.
 Preoperative optimization of patients is requisite – smoking cessation, weight loss if indicated, and nutritional restitution.
Preoperative optimization of patients is requisite – smoking cessation, weight loss if indicated, and nutritional restitution.
 Autologous techniques are ideal in reconstruction, utilizing muscle and fascia – utilizing separation of components and fascia lata grafts.
Autologous techniques are ideal in reconstruction, utilizing muscle and fascia – utilizing separation of components and fascia lata grafts.
 Prosthetic meshes and bioprosthetics may be utilized in addition to, or in lieu of, flaps for recalcitrant cases.
Prosthetic meshes and bioprosthetics may be utilized in addition to, or in lieu of, flaps for recalcitrant cases.
 Advanced techniques may include tissue expanders, laparoscopic methods, free flaps, and even abdominal wall transplantation.
Advanced techniques may include tissue expanders, laparoscopic methods, free flaps, and even abdominal wall transplantation.
 Postoperative management begins in the intensive care unit (ICU) and continues into postoperative rehabilitation.
Postoperative management begins in the intensive care unit (ICU) and continues into postoperative rehabilitation.
Introduction
The history of abdominal wall reconstruction is ensconced in the surgery-in-general literature. Abdominal wall hernias, which occur either congenitally (e.g., umbilical) or in an acquired fashion (e.g., postpartum, posttrauma, and postsurgery or iatrogenically), are routinely treated by general surgeons. Plastic surgery involvement in abdominal wall reconstruction came to the fore in complex cases where routine techniques exploiting local tissues and mesh would not suffice. Plastic surgery consultation became indicated for flap mobilization, regional tissue transfers, free flaps, and tissue expansion techniques. A seminal paper by Ramirez et al.1 revisited and popularized components separation as a useful technique in abdominal wall reconstruction. What has followed is an understanding of abdominal wall muscular dynamics, the role of component separation and muscle realignment as a central tenet of any reconstruction, and advances such as endoscopic or minimally invasive methods to perform component separation. Recent advances include perforator-sparing techniques, and evolution in biologic as well as synthetic meshes.
Most surgeries are performed in the rich world – the world’s wealthiest 2 billion people get 75% of all the surgery done each year while the poorest 2 billion only get 4%.2 With increasing access to care, parts of the developing world will face similar challenges of abdominal wall reconstruction.
The ascendancy of the evidence-based medicine paradigm over the last two decades has focused attention on both the short-term failure rates of techniques as well as the long-term data. A landmark paper by Luijendijk et al.3 showed that, if followed for 10 years, the failure rates with primary suture techniques are 2 in 3, and with synthetic meshes 1 in 3. This is unacceptably high. Consequently, there has been an escalation in interest and an introduction of novel techniques in addressing these problems, as long-term data highlight the inadequacy of previous methods. The influence of evidence-based medicine has driven a resurgence of investigations into abdominal wall reconstruction techniques, as well as multispecialty collaboration between general surgeons, colorectal surgeons, trauma surgeons, minimally invasive surgeons, and plastic surgeons.
Historical perspective
The components separation method
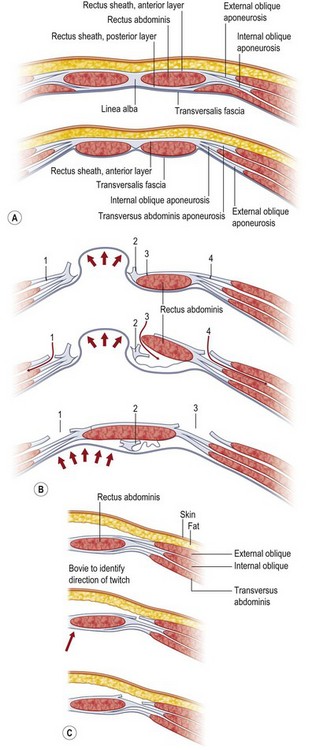
The technique most credited for the advancement in approach to abdominal wall reconstruction using autologous methods is the components separation method, as popularized in the modern plastic surgery literature by Ramirez et al. in 1990.1 Demonstrating clinical vignettes and elegant cadaver dissections, this manuscript advanced the technique of using bipedicled, neurotized, musculofascial advancement of the rectus abdominis muscles and internal oblique with transversus abdominis muscles. Historically, however, this technique had been mooted as early as 1916 by Gibson,8 then again in 1929 by Dixon,9 and finally in 1961 by Donald Young.4 Young described separating the transversus/internal oblique/rectus unit from the external oblique, similar in principle to components separation.
Components separation is a more nuanced way of performing the classic trauma teaching of lateral relaxing incisions. By laying bare the anatomy of the abdominal wall, Ramirez et al. showed that releasing the rectus abdominis muscle at the linea semilunaris from the external oblique muscles creates an avascular plane between the external oblique and the rectus–internal oblique–transversus complex.1 Since the segmental intercostal innervation to the rectus abdominis muscle arises deep to the internal oblique musculature, this technique preserves not only the vascularity but also the innervation to the muscle, which is principal in a stable reconstruction. Blood supply to the rectus abdominis muscle arises from segmental intercostals, as well as from the superior epigastric artery as it becomes the terminus of the internal mammary artery. The dominant blood supply is from the deep inferior epigastric artery (DIEA) as it arises from the external iliac artery.
In 2000, Shestak et al.5 further described the components separation method and detailed its limitations. The surgical goal is to recreate the midline tendon (linea alba) where the paired rectus muscles are held to length, and the relentless unopposed lateral traction of the external oblique, internal oblique, and transversus complex is paired to the contralateral complex. The authors describe incising the fascia 1 cm lateral to the linea semilunaris, identifying the plane between the external oblique and internal oblique fascia (Fig. 12.1). They underscore the importance of dissecting superior to the internal oblique fascia, thus protecting the underlying nerves to the rectus abdominis muscle, and avoiding injury to the spigelian fascia (reducing the likelihood of a spigelian hernia). Extent of lateral dissection is determined by the amount of laxity present, with maximal advancement of each ipsilateral complex toward the midline of 4 cm in the upper abdomen, 8 cm at the waist, and 3 cm in the lower abdomen. An additional 2 cm can be advanced with elevation of the rectus muscle off the posterior rectus sheath (see Fig. 12.9, below).
As previously mentioned, the dominant perfusion of the soft tissues and skin of the anterior abdomen is the territory of the DIEA as well as contributions from the superficial inferior epigastric artery and from the intercostals. A notorious complication of components separation is marginal or incisional necrosis of the soft-tissue pannus, a result of devascularization of the anterior abdominal soft tissues by dividing the perforators that course through and perforate the rectus abdominis muscles. Several techniques have been advanced to minimize the risk of ischemic complications.10
Restoring abdominal wall integrity in contaminated tissue-deficient wounds using autologous fascia grafts)
The tensor fascia latae (TFL) technique has been described and utilized as a pedicled or free flap, as well as a nonvascularized graft, and has been shown to revascularize, incorporate, and restore peritoneal continuity (of substantial importance in patients with Crohn’s disease and extensive adhesive disease). In addition, in the event of a repeat laparotomy, it can be reincised and subsequently reapproximated, and expected to heal, similar to native fascia.11
As a pedicled flap, the TFL musculocutaneous unit is vascularized based on the transverse branch of the lateral femoral circumflex artery. A type I muscle by the Mathes–Nahai classification, its arc of rotation can treat composite defects of the infraumbilical abdominal wall. A free TFL muscle or musculocutaneous flap is more versatile and can replace large defects (up to half of the anterior abdominal wall) anywhere in the abdomen. The free flap is typically anastomosed to intraperitoneal vessels such as the right or left gastroepiploic artery on the greater curvature of the stomach, omental vessels, or regional vessels in the musculature such as the superior epigastric or DIEA.12
Consistent with established principles, autologous tissue-based repairs have been shown to expedite wound healing and outperform traditional synthetic mesh-based techniques in contaminated, tissue-deficient abdominal hernias.13
Rise of the biologics – seeking the holy grail of ideal repair material
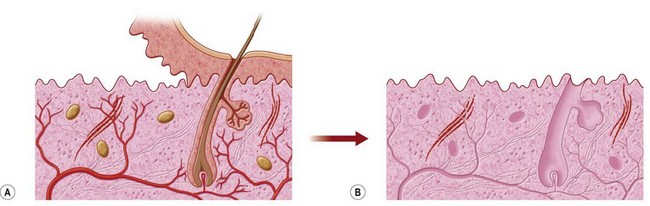
Those characteristics are potentially obtainable in many biologic meshes, which are sourced from acellular dermal matrices (human, porcine, bovine), or other large sheeted xenogeneic sources such as acellular bovine pericardium. Processing involves making these materials acellular to decrease the risk of rejection, as well as enzymatically cleaving the xenogeneic epitopes that could cause a cross-species reaction in biologic meshes derived from nonhuman sources (Fig. 12.2). Discussion of the available types of mesh is beyond the scope of this chapter.
Primary closure versus synthetic mesh repair
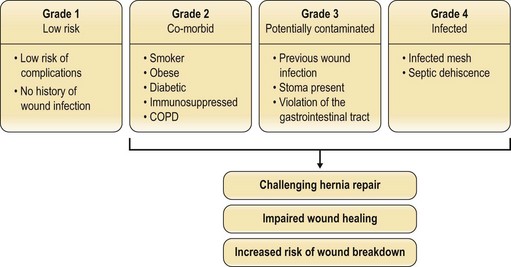
Factors such as body mass index (BMI), smoking status, nutritional status, and the presence of comorbidities (e.g., diabetes mellitus, chronic obstructive pulmonary disease (COPD)) have been investigated to see which predispose to postoperative herniation.14 The patients at highest risk of recurrence have an infected mesh or dehiscence from a septic cause. Potentially contaminated wounds (stoma, prior infection, enterotomy) are inherently higher-risk (Fig. 12.3). The problem remains multifactorial. Whether running or interrupted sutures are used, whether permanent or slow-resorbing sutures are employed, whether figure-of-eight knots or simple knots are put down, none has been conclusively shown to have a significant outcome difference.
When hernias are repaired, the long-term success of these techniques is surprisingly low. The authoritative papers by Luijendijk et al. in 20003 and by Burger et al. in 200415 indicate that, while mesh is superior to primary suture technique, even mesh repair over a 10-year follow-up period has a 32% failure rate. Numerous causes are thought to contribute to these findings – cyclical fatigue of the synthetic mesh device by constant respiratory excursion of the abdomen, lack of true bioincorporation into the mesh, or biofilm seeding leading to capsular contraction, encapsulation, and eventual extrusion.
Evidence-based medicine is now de rigueur when addressing almost any clinical concern. No longer satisfied with anecdotes, the savvy clinician demands the data – and it is this critical scrutiny that has highlighted the deficiencies of time-honored techniques and opened the surgical community to challenge the status quo. Collaboration and cross-pollination of techniques between the general surgery and plastic surgery disciplines have led to greater insights than each could have individually. The type of material used – acellular cadaveric dermis (from human or animal source) or different types of mesh (polypropylene, soft polypropylene) – remains a topic of debate in the literature, with studies revealing favorable results with either material.16,17
Basic science/disease process
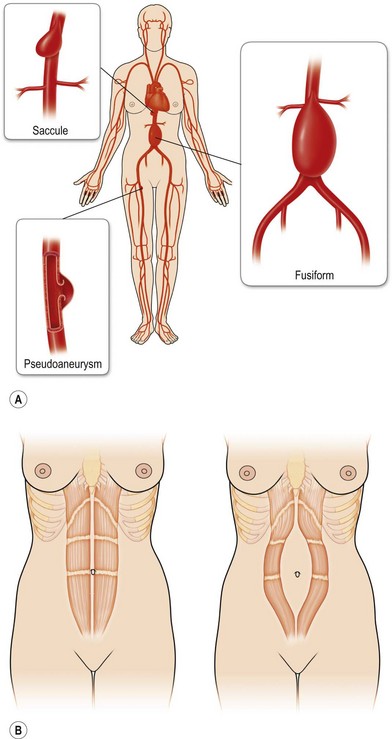
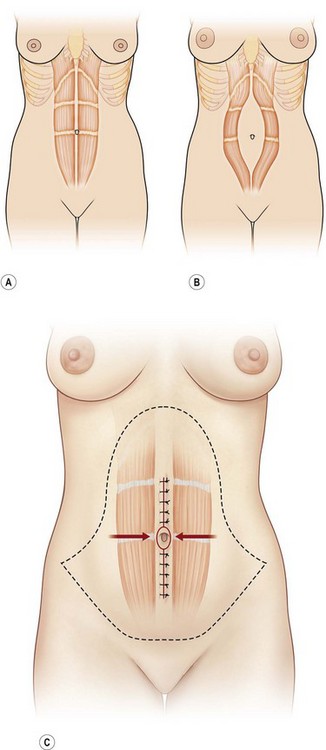
Rectus diastases are not true abdominal wall fascial defects but are pathological stretching to the linea alba either congenitally or, most frequently, postpartum. Functionally, a diastasis is analogous to an aneurysm – wherein the adventitia (fascia) and intima (peritoneum) are intact but the muscular layer is absent (Fig. 12.4). In techniques for abdominal wall reconstruction that do not centralize muscle, or cases in which the abdominal muscles have retracted beyond the possibility to be reapproximated, a functional diastasis remains, in lieu of the tendinous fusion of the paired rectus abdominis muscles. This diastasis can enlarge over time from intra-abdominal pressure, even to the point of requiring repair. Repair is achieved without intraperitoneal entry, by plicating or imbricating the defect so that the rectus abdominis muscles are returned to the midline (Fig. 12.5).
A review of the long-term data reveals that Luijendijk et al. reported 43% versus 24% for initial recurrences and 58% versus 20% for secondary recurrences at 3 years.3 At 10-year follow-up, Burger et al. reported 63% versus 32% recurrence if primary suture techniques were used versus prosthetic mesh.15 Despite the reduction in recurrence rates, the rates remain unacceptably high.
The development and adoption of prosthetic materials have made a tremendous impact on the treatment of hernias. Synthetic mesh was introduced in the 1950s and has become a common component of the repair process. Data from the largest prospective, randomized, multicenter study show a significant decrease in recurrence rates in relatively healthy patients with small defects (<6 cm length or width) who received synthetic mesh versus those repaired primarily with suture alone.3