Introduction
The need for soft tissue coverage in abdominal wall reconstruction implies a lack of tissue beyond the availability of local tissue to be recruited to resurface the defect. As the majority of abdominal wall defects can be reconstructed with the redundant tissue from the torso, these defects represent a more complex subset of abdominal wall reconstructions. Indications for flap coverage vary by etiology of defect, defect characteristics, and timeline for closure. Multiple clinical scenarios can lead to a loss of abdominal wall soft tissue requiring flap reconstruction, including oncologic resection, traumatic injury, radiation-associated wounds, skin necrosis, soft tissue infection, and septic evisceration.
The amount of soft tissue loss and amount of coverage able to be performed with local skin advancement must be factored into the reconstructive plan. Abdominal wall defects requiring soft tissue coverage can be classified as partial thickness defects, involving the skin and subcutaneous tissue only, or full thickness composite defects which involve loss of the abdominal wall musculofascia in addition to the overlying skin and subcutaneous tissue. The indications for soft tissue replacement in abdominal wall reconstruction also depend on the chronicity of the wound/defect with some defects benefiting from early flap coverage, others from delayed flap coverage, while some defects might be better served with chronic wound care and healing by secondary intention.
Indications
Historically, abdominal wounds were treated with wound care and allowed to heal over time by secondary intention or were reconstructed with a skin graft after the local wound environment was optimized. This resulted in a prolonged course of care and significant morbidity. In time, the concept of delayed-primary closure gained popularity allowing certain patients with favorable wound characteristics to undergo closure after a short period of wound care, instead of being committed to weeks or months of open wound care. This enabled patients to achieve definitive wound closure without a skin grafted surgical site and associated donor site morbidity.
Soft tissue flap reconstruction offers significant advantages over wound closure. Flap reconstruction is performed in a single stage procedure obviating the need for chronic wound management. Flap reconstruction offers immediate and definitive wound closure mitigating the local milieu of inflammatory response and local tissue injury. In reconstructions involving musculofascial reinforcement with bioprosthetic mesh, these two factors are critical. When bioprosthetic mesh is interposed between two well vascularized tissue planes (posterior abdominal wall/peritoneal cavity and a soft tissue flap superficially) bidirectional vascular ingrowth can be achieved accelerating the period of bioprosthetic mesh revascularization and incorporation. In addition, a closed wound environment diminishes the proinflammatory state of an open wound, which limits the degree of enzymatic degradation of the bioprosthetic mesh during the incorporation phase.
Over recent years, the role of negative pressure wound therapy (NPWT) has revolutionized the approach to wound care particularly in the abdominal wall. NPWT allows preservation of the wound environment by managing fluid losses, decreasing bacterial contamination, and accelerating granulation tissue formation. In abdominal wall reconstruction, this translates in preserving the option for delayed closure by flap reconstruction or delayed primary closure.
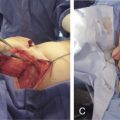
Composite full-thickness loss of the abdominal wall musculofascia and overlying soft tissue represents the most complicated abdominal wall reconstructions, sometimes requiring multiple staged reconstructive procedures. Re-establishment of myofascial continuity is paramount to setting the stage for a durable abdominal wall reconstruction. Reconstituting the deficient myofascia with an inlay of bioprosthetic mesh converts the open abdomen to a more manageable abdominal wall wound. For midline defects, early abdominal closure with primary rectus musculofascial reapproximation over bioprosthetic mesh provides superior outcomes to bridging the fascial defect with bioprosthetic mesh. The risk of developing a hernia increases seven-fold when bridging fascial repairs are performed instead of reinforced mesh repairs. All attempts should be made to achieve fascial coaptation as bridging repairs are far more likely to develop hernias in the early postoperative period <18 months. When early fascial closure is not an option owing to ongoing debridement of the myofascia or the need to perform a second-look laparotomy, a temporizing abdominal wall closure can be utilized, such as the NPWT system. A static bridging wound dressing protects and insulates the viscera while controlling fluid loss in the wound bed. NPWT also provides abdominal stability in the early postoperative period for patients undergoing mechanical ventilation and later when they ambulate and undergo physical therapy.
The goals of abdominal wall reconstruction of a full-thickness composite defect are to re-establish the integrity of the myofascial layer and provide cutaneous coverage. The prevailing strategy in full-thickness composite reconstruction of the abdominal wall is to avoid patching the defect and instead reinforce the entire surrounding section of abdominal wall. This is accomplished through inlay (intra-abdominal) mesh placement with the mesh sutured to points of fixation: innervated myofascia, lamellar aponeurotic tissue, or bone. Surgical planning in abdominal wall reconstruction must address local wound conditions, including bacterial contamination, previous operations, presence of ostomies, and prior radiation therapy, all of which can increase the risks of compromised wound healing and surgical site infections. Composite abdominal wall defects often involve significant loss of innervated myofascia and overlying skin in a dimension that is greater than the surrounding tissue’s ability to be recruited and mobilized for closure. In such cases, regional or distant tissue flaps must be used for closure, and the resultant repair will no longer be dynamic and coordinated with the remaining abdominal wall musculature, unless functional muscle transfers are performed.
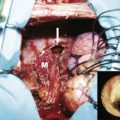
When both soft tissue and musculofascia require reconstruction, such as a composite defect, it is preferred to reconstruct these two components with different techniques. Mesh and often component separation is used for the musculofascial component and soft tissue flaps are used for the soft tissue defect. The use of the fascial component of a flap for musculofascial reconstructions can result in increased bulge or hernia. In addition, insetting the fascial component of a fasciocutaneous flap to reconstitute the myofascial defect can potentially compromise the vascularity of the overlying skin island by placing undue tension across the myofascial repair. Thus, for composite midline defects, myofascial reconstruction is generally performed with component separation, with the use of mesh, to ensure the structural integrity of the repair. Both synthetic and bioprosthetic mesh materials have been used. Surgeon preference and the variables of any given clinical scenario will determine whether bioprosthetic mesh or synthetic mesh is implanted. Regardless of mesh type, the expectations are that the mesh will maintain the abdominal wall contour, without development of a hernia or bulge. In addition, the mesh should be able to interface with the intra-abdominal viscera without forming extensive adhesions or erosion that can lead to fistulization. Bioprosthetic and synthetic meshes can meet these expectations, and the decision to use either is based on patient comorbidities, wound contamination, prior radiation, availability of greater omentum, and the quality of the overlying soft tissue.
Anchoring the mesh to stable fixation points in the abdominal wall is a key element of a successful repair. The abdominal wall pillars are stable fixation points; these include the costal margin and rib superiorly, the linea semilunaris and linea alba anteriorly, the inguinal ligament and iliac crest inferiorly, and the investing lumbar and paraspinal fascia posteriorly. To achieve a stable repair, it is important to create an inlay mesh inset that links these anatomic structures.
Whenever feasible, a component separation should be considered to either reduce the tension on a myofascial repair to facilitate a second layer fascial closure over a biologic mesh inlay or to advance myofascial flaps to reduce the diameter of a bridging mesh repair. Minimally invasive component separation (MICS) techniques can be utilized to achieve myofascial release and closure under physiologic tension while decreasing seroma formation and preserving perfusion to the midline skin. Key technical aspects of minimally invasive component separation include the creation of 3 cm wide, subcutaneous access tunnels over the anterior rectus sheath from the midline to the semilunar line at the level of the costal margin. Through these access tunnels, the external oblique aponeurosis is vertically incised 1.5 cm lateral to the linea semilunaris. A narrow (2.5 cm wide) subcutaneous tunnel is created with electrocautery superficial to the external oblique aponeurosis over the planned release location using a narrow retractor and a headlight. The external oblique aponeurosis is now released approximately 1.5 cm lateral to the lateral edge of the rectus sheath from 12 cm above the costal margin superiorly to near the pubis inferiorly. Next, lateral dissection between the internal and external oblique muscle is performed to the mid-axillary line. Subcutaneous skin flaps are then elevated over the anterior rectus sheath circumferentially to the medial row of rectus abdominis perforator vessels. Next the musculofascial edges are advanced and reapproximated over the mesh with sutures placed through the musculofascial layers and mesh. A clinical controlled study demonstrated that the minimally invasive technique patients had significantly fewer wound healing complications (32% vs 14%, p = 0.026) and skin dehiscence (28% vs 11%, p = 0.01) than the traditional open CS group. These improved wound healing outcomes are likely due to preservation of the vascularity of the overlying skin flaps and reduction of paramedian dead space, which is the surgical principles highlighted in the MICS procedure.
The reconstructive algorithm for skin coverage of full thickness abdominal wall defects begins with local skin advancement flaps and expands to local perforator flaps, regional pedicled flaps, and ultimately free flap reconstructions. The overlapping angiosomes of the abdominal wall’s cutaneous blood supply allow for wide undermining and skin advancement. In addition, tissue expansion can be performed in the trunk to increase the surface area and availability of local fasciocutaneous flaps as an alternative to a pedicled or free flap donor site. In cases of prior radiation, prior surgery, or excessive skin resection, a pedicled regional or free flap may be required to provide adequate soft tissue coverage. Composite abdominal wall defects can involve significant loss of innervated myofascia and overlying skin in a dimension that is greater than the surrounding tissue’s ability to be recruited and mobilized for closure. In such cases, regional or distant tissue flaps must be used for closure, and the resultant repair will no longer be dynamic and coordinated with the remaining abdominal wall musculature.
Preoperative Considerations
Preoperative planning for abdominal wall defects that require flap reconstruction must factor defect type, defect location, availability of surrounding soft tissue and in certain cases, planned intra-abdominal reoperation. As with any reconstruction, there is plan A and an algorithm that plans for all contingencies (i.e., plans B through Z). This plan with multiple back-up plans is critical in abdominal wall flap cases, as often the back-up plan becomes the plan and time cannot be wasted deliberating each step in the reconstructive algorithm. Special consideration must be given to flap type (muscle, myocutaneous or fasciocutaneous); donor site (one that is harvested supine or requires a position change); recipient vessels and the potential need for vein grafts. In addition, flap reconstructions on the trunk merit discussion of postoperative positioning and mobility restrictions. This begins during flap inset to ensure postoperative positioning or dependent pressure/swelling will not compromise flap perfusion. As with any flap, a plan for postoperative monitoring must be established. Flap type and practice pattern will determine the method of monitoring, clinical exam, Doppler (handheld vs implantable), tissue oximetry, tissue temperature, or vascular flow monitors.
Patient Evaluation
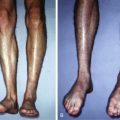
When evaluating a patient for abdominal wall flap reconstruction, history and physical examination, laboratory assessment, as well as consultation from other services including general surgery, internal medicine, pulmonology, and nutrition are critical for successful operation. Features within a history such as smoking status and alcohol abuse are important due to the impaired wound healing effects associated with vasoconstriction and immunocompromise. In addition, aspects of a patient’s past medical history such as peripheral vascular disease, diabetes mellitus, and autoimmune disorders can be associated with impaired wound healing. Prior abdominal surgery can alter perfusion to the skin and musculofascia. Previous surgery also causes visceral adhesions that when complex can lead to unplanned enterotomy which can complicate reconstruction efforts. Additionally, prior radiation exposure can decrease wound healing capacity, which can lead to a higher complication rate in radiated abdominal wall reconstructions.
Physical examination focuses on characteristics of the abdominal wall defect, including chronicity; partial vs full-thickness defects; size and location of the defect; wound contamination; presence or absence of an ostomy; and the presence or absence of enterocutaneous fistulae. Accordingly, the size and location of the defect can help determine future surgical management, as well as which type of flap will be required, local, regional, or free flap. Preoperative evaluation should focus on factors such as body mass index, abdominal wall laxity, and evaluation of potential donor sites for flap coverage. Weight reduction for obese patients and tobacco cessation for active smokers are recommended to decrease complication rates, whenever feasible. For elective reconstructions smoking cessation for 8 weeks prior to surgery and targeted weight loss to individual patient goals, will reduce 30-day morbidity in abdominal wall reconstruction. Physical examination should focus on the potential donor sites for flap harvest circumferentially around the defect.
As with any intra-abdominal procedure, the patient’s metabolic and hematologic status should be evaluated prior to surgical intervention. As such, a complete metabolic panel, complete blood count with differential, and a hepatic function panel should be performed to evaluate any electrolyte disturbances, anemia, infectious processes, or hepatic disturbances. Electrolytes replacement is of particular importance in patients with enterocutaneous fistulae or open chronic abdominal wounds. Nutritional status should be optimized preoperatively due to the fact that poor nutritional status is associated with impaired wound healing and impaired immune response. Nutritional status can be assessed via measurements of albumin and prealbumin and should be included with routine laboratory evaluations for potential abdominal wall reconstruction candidates.
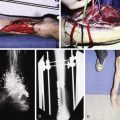
Abdominal CT scan is used to identify the extent of the abdominal wall defect and the integrity of adjacent musculofascial structures and aid in preoperative planning. CT angiography can be used to delineate the vascular anatomy of the abdominal wall. CT angiography allows the surgeon to refine plans for flap selection and make operative decisions preoperatively. CT angiography is invaluable in accurately identifying the abdominal wall vasculature to aid in identifying recipient vessels when planning a free tissue transfer. This is of particular importance in patients who have undergone prior abdominal surgery where the vascular anatomy can be altered significantly and flap perforators can be difficult to localize and recipient vessels may have been injured at previous surgery.
Overview of Reconstruction by Region
The anterior abdominal wall can be divided into three anatomic regions: the epigastrium, the periumbilical region, and the hypogastrium ( Tables 18.1–18.3 ). The relationship of defects to these anatomic regions guide decision-making when regional pedicled flaps are planned for reconstruction. Options for pedicled flaps in the upper abdomen include latissimus dorsi and omental flaps. Thigh-based flaps such as anterolateral thigh, vastus lateralis, and tensor fascia lata flaps are generally able to reach the hypogastrium and flank as pedicled flaps. If a pedicled flap is not available or feasible, a thoracoepigastric bipedicled fasciocutaneous flap may provide a local tissue alternative in patients who are not candidates free tissue transfer.
| Local | Pedicled | Free | |
|---|---|---|---|
| Epigastric | Transposition: IM, IC, SE | Rectus | Thigh-based: ALT, AMT, VL, TFL, RF, STF |
| Keystone | Omentum | Back-based: LD, TAP, Scap/Para | |
| Bipedicled fasciocutaneous | Thigh-based |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree