Abdominal Wall Reconstruction
Gregory A. Dumanian
INTRODUCTION
Abdominal wall reconstruction (AWR) is a proving ground for the principles of plastic surgery. It requires a thorough knowledge of anatomy, an understanding of the physiology of the intra-abdominal viscera, the manipulation of multiple tissue types, the handling of alloplastic materials, and wound care. The quality of the reconstruction is judged both by the durability of the abdominal muscle repair and on the aesthetics of the final draping of skin and soft tissues over the abdominal wall. According to the Cochrane database, as many as 11% to 53% of all midline laparotomies will result in a hernia. Challenges facing surgical management of hernias include the obesity epidemic and the advent of minimally invasive procedures that have eroded the familiarity of other surgery disciplines with large open procedures and complex wounds.
This chapter attempts to provide the reader a framework for the management of all types of abdominal wall situations, including wounds, fistulae, and hernias. Management of the abdominal wall depends on:
An understanding of abdominal wall physiology and the forces on the abdominal wall that lead to hernia formation.
Strategies to deal with complex abdominal wall wounds and fistulae.
An appreciation of factors, such as prior surgical history, bowel issues, and nutrition, that play a role in the timing and sequence of operative procedures.
The attention to skin vascularity during hernia repair.
FORCES ON THE ABDOMINAL WALL
The abdomen can be conceptualized as a pressurized cylinder. The posterior one-third of the cylinder is rigid. With inspiration or for motion of the upper body and arms, a combination of diaphragm descent and abdominal wall muscle contraction causes an immediate rise in intra-abdominal pressure. In a healthy abdominal wall, the increased internal abdominal pressure is matched by an increase in the tone of the abdominal wall muscles. Where there is a local imbalance of intra-abdominal pressure and muscle tone, a bulge becomes apparent. Examples of bulges include the linea alba with the condition of rectus diastasis after childbirth and the lateral bulges seen not infrequently after flank incisions. What is important is the uniformity of the abdominal wall counterpressure. When this uniformity of abdominal wall counterpressure is lost, bulges and hernias emerge. Episodic high peaks of intra-abdominal pressure caused by chronic coughing and periodic lifting of heavy objects hasten the deformation of the weak area of the abdominal wall by the mobile internal viscera. Whereas bulges are comprised of some aspects of intact (though weakened, partially resected, or denervated) abdominal wall, true hernias are contained only by scar. The physiologic importance of this difference is understood by observing the cross-sectional appearance of bulges that are smooth curves, in comparison to the omega shape of ventral hernias. Bowel can become caught and strangulate on the lip of a hernia, while there are no risks of incarceration with bulges. While the medical indication to repair hernias is the prevention of bowel obstruction and the improvement of localized pain, the indication to repair bulges rests solely on the issues of pain that can occur with tissue stretching. Hernias typically expand with time, due to the tendency of scar to stretch and deform, and therefore often do not reach a steady state. Bulges, on the other hand, can reach a steady state in size when the inelasticity of the tissue is matched to the decrease in abdominal wall pressure that would accompany an increase in intra-abdominal volume.
Obesity plays a role in two ways–first, there is an increased amount of tissue inside the abdominal wall raising baseline intra-abdominal pressure. Second, the abdominal wall must support a greater amount of weight above the diaphragm, increasing both the intensity and number of peaks of high intra-abdominal pressure. Each peak of pressure causes stress at the suture-tissue interface.
ABDOMINAL MUSCLE PHYSIOLOGY
In a normal abdomen without a hernia, downward descent of the diaphragms and abdominal muscle contraction creates elevated intra-abdominal pressure. The Valsalva maneuver is but one example of the body using elevated intra-abdominal pressure to brace and make more rigid the torso for effective use of the upper body and arms for lifting. Abdominal muscle contraction in this instance is predominantly isometric–meaning that the muscle fibers increase their tone but without sarcomere shortening. In cases of large hernias, abdominal muscle contraction no longer increases intra-abdominal pressure, because the viscera can escape out into the hernia. The abdominal wall muscles now shorten (isotonic contraction) rather than simply increase in tension. This increases the work of the abdominal wall, because isotonic contraction consumes more energy than does isometric contraction. Additionally, the diaphragm and upper torso no longer can “push off” against a pressurized abdomen, creating dysfunction between the chest and abdominal compartments. The more massive the hernia, the larger the derangement of abdominal wall physiology.
Another concept useful in understanding the forces of the abdominal wall musculature and the utility of AWR is abdominal wall compliance. Compliance is measured by the change in volume for a given change in intra-abdominal pressure. As abdominal compliance increases, more volume can be accommodated for the same increase in pressure. If the compliance of the abdominal wall is improved, it follows that during a hernia repair, the contents of a hernia sac (volume “outside” the abdomen) can be more easily reduced back into the abdomen. Indeed, it has been shown that experimental hernia repairs are more successful when the abdominal wall is compliant than when it is stiff. Causes of abdominal wall stiffness include lateral incisions, large abdominal meshes from prior hernia repair, and intraperitoneal sepsis and scar formation.1 An emphasis on the forces on the abdominal wall is more important for surgically induced ventral hernias than for spontaneous abdominal wall defects, where deficiencies in extracellular matrix may play a prime pathologic role.2
The Effect of Repair on Abdominal Wall Forces
The goal of a hernia repair is to reestablish uniform abdominal wall counterpressure against the viscera, improving the counterpressure where it is weak, and if necessary, weakening the abdominal wall where it is strong. In suture repairs (also known as direct repairs), the abdominal wall is approximated primarily. There is no change in the abdominal wall compliance, and the greatest tension is at the site of the repair. “Unsupported” direct repairs (those without some type of mesh) rely solely on sutures to hold the abdominal wall. “Supported” direct repairs attempt to distribute the forces on the repair over a larger area by adding mesh to the repair site. Another type of hernia repair is with a piece of mesh that spans across an open defect of the abdominal wall. In these types of “bridged” repairs, sutured mesh acts like a cap or lid, replacing the weak area of the abdominal wall. This avoids an increase in focal forces on the abdominal wall where the tissues have previously failed. The strength of the mesh to resist outward deformation depends on the strength of the circumferential attachment of the mesh to the normally innervated abdominal wall. The larger the hernia, the further the mesh center is from innervated abdominal wall, and the greater will be the eventration.
The prime reason for hernia recurrence is suture pulling through the tissues over time like a wire cutting through ice. Improved force distribution over the hernia construct with decreased tension experienced by each stitch will lead to lower suture pull-through. Direct supported repairs use mesh as a load-sharing manner as opposed to a load-bearing manner for bridged repairs. Improved force distribution and decreased pull-through are the primary reasons that supported repairs have lower failure rates than primary repairs or bridged repairs. Obesity and lateral abdominal wall noncompliance increase the forces felt by each stitch–an explanation for higher failure rates in these situations.
A final manner of repairing the abdominal wall is with the components separation technique for midline defects. Releases of the external oblique muscle and fascia from its attachment to the rectus abdominis muscles allows for a repair of the rectus muscles in the midline while simultaneously improving the abdominal wall compliance on the sides. Components’ separation repairs can be either “unsupported” or “supported” depending on the clinical situation.
CLOSING THE WOUND
When patients are ill, wounds are inflamed, and nutrition is poor, open abdominal wounds should be treated with simple procedures that have high chances for success. Patients who are packed open after a laparotomy can often be closed primarily after bowel swelling has resolved. For those patients that cannot have their fascia closed due to persistent visceral swelling or intra-abdominal sepsis, early wound closure in the simplest manner possible provides multiple benefits, including patient comfort, ease of wound care, and a decreased incidence of enterocutaneous fistulae.3 To devise a wound closure plan, the following questions must be answered: Are the viscera “frozen,” and what are the chances for an evisceration? Do bowel contents need to be controlled? What are the location, size, and characteristics of the wound? Should the surrounding skin be modified to help achieve wound closure?
Open Wounds and Evisceration
Bowel found outside the skin are surgical emergencies that require immediate evaluation and thoughtful treatment in the operating room. Much depends on the cause of the loss of the abdominal wall integrity. Pure technical problems of broken suture and untied knots do occur, but are uncommon. When detected quickly, these patients at exploration have pristine wounds and can simply be reclosed. If sutures are noted to have torn out of weak fascia, the conversion to a direct supported repair or the use of retention sutures is successful without repeat disruption 55% of the time.4 Mass closures with retention sutures can be successful, but the sutures can cause skin and tissue necrosis.
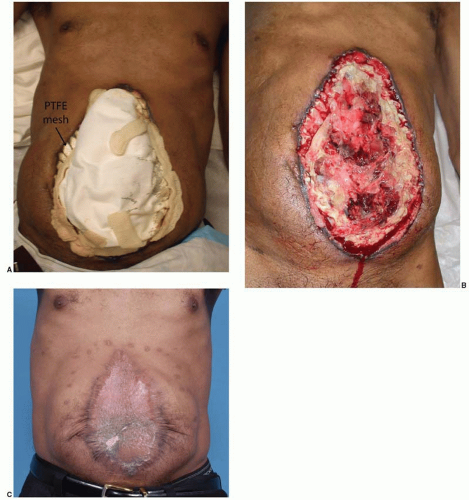
More commonly, the patient has an ileus and the bowel is swollen. These are usually contaminated wounds and there is often an underlying septic intra-abdominal process. Therefore, the goal of surgery is to replace the intestines back into the abdomen and to prevent a second evisceration. Necrotic tissue is debrided, intra-abdominal fluid collections are allowed to drain, and a compartment syndrome from swollen bowel is avoided. For these sick patients, a temporary mesh–typically absorbable polyglycolic acid–is placed using a running absorbable monofilament suture to “close” the abdominal wall and to keep the viscera in their proper domain (Figure 93.1A). The bridging nature of the mesh across the fascial defect increases the intra-abdominal volume. Secondary dehiscence is unusual because the lateral abdominal muscles are now shortened and cannot generate a maximal contraction during coughing and movement. The porous nature of the closure allows intra-abdominal fluid to drain into the overlying gauze or a subatmospheric pressure dressing. When the patient has stabilized, skin closure is performed by delayed primary closure, by skin grafts, or by secondary intention. When the skin gapes widely and several months would be required for closure by secondary intention, skin grafting provides the simplest and most reliable closure as discussed below.
An alternative to closure with a temporary porous mesh is patching the open fascial defect with a human or porcine bioprosthetic mesh. Bioprosthetic meshes have been touted for their tolerance of inflamed fields, resistance to infection, and ability to restore abdominal wall continuity, at least temporarily. While this may be true, granulation of the bioprosthetic meshes may lead to a rapid loss of tensile strength of the biomaterial. Disadvantages of these products include their high cost, and relative impermeability to intra-abdominal fluid in comparison to polyglactin mesh. These disadvantages would be less important if a later AWR could be avoided, but this has not yet been borne out in the literature. Finally, repair of these fascial defects with permanent mesh was tried and abandoned in the earliest papers on AWR. The heavyweight polypropylene was associated with fierce adhesions, extrusions, enterocutaneous fistulae, and occasional mortality.
While eviscerations with exposed bowel require operative intervention, the treatment of open wounds after laparotomy requires a more thorough history, physical examination, and radiologic evaluation. Open wounds after laparotomy may represent simple skin wounds, but they may also harbor fascial defects with exposed viscera at their base. Clues for fascial dehiscence include loose abdominal sutures at the base of the wound, a history of a “seroma” (a clue that intra-abdominal fluid is emerging through the open abdominal wall), or a computed tomography (CT) scan demonstrating superficial bowel loops. The timing from the latest abdominal exploration is also a critical factor in the evaluation of a potential evisceration. Open wounds with a fascial dehiscence less than 1 week from the last exploration are at high risk for evisceration and should probably be explored to prevent an even larger dehiscence leading to a surgical emergency. Wounds with fascial dehiscence over 2 weeks from laparotomy usually have enough intra-abdominal adhesions to avoid an evisceration and can usually be managed as standard wounds. Wounds between 7 and 14 days require judgment to decide whether the exploration would have a higher chance of causing a bowel injury than it would prevent an evisceration. Patient factors such as age, previous presence of adhesions, and wound healing issues such as steroids play into the decision. Patients on steroids
may require up to 3 weeks before adhesions between bowel loops are strong enough to avoid an evisceration.
may require up to 3 weeks before adhesions between bowel loops are strong enough to avoid an evisceration.
For the patients with open wounds, unknown fascial integrity, and a low chance for evisceration, informed consent is important. Patients with open abdominal wounds after a laparotomy have a high incidence of later hernia formation. These patients should be informed that surgical treatment of open abdominal wounds have a risk of creating an enterocutaneous fistula, and that with or without treatment, there is a high risk of a ventral hernia. The patients are informed that “conservative” treatment with dressing changes is also not without risk, as the intense local inflammation may cause an opening at a bowel suture line or site of a previous serosal tear. All things considered, early wound closure increases patient comfort, reduces the chances for bowel injury, and is the first step in AWR. The most reliable method of wound closure is with skin grafts (Figure 93.1B and C). The “two-dimensional” healing of skin grafts is not dependent on the patient’s nutrition, unlike the “three-dimensional” healing required for sutured skin flaps, and may proceed despite suboptimal nutrition parameters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree