Childhood LAD may have previously been more common in the UK as there seems to have been no difficulty in dermatologists collecting these cases in the past [14,23]. In contrast, in the last 15 years there have been only five cases of childhood LAD in Oxfordshire and Buckinghamshire (approximate population 1,200,000). This geographical variation may be attributable to an infectious trigger for the disease; however, this is speculative. The occurrence within a single ethnic group in South Africa suggests that there may be a genetic susceptibility in certain racial groups.
Pathology.
The single most important investigation is a skin biopsy of uninvolved skin for direct IF [62]. A biopsy should be performed in all suspected cases because it is virtually impossible to differentiate this disease from other autoimmune bullous diseases such as bullous pemphigoid and DH with any degree of diagnostic certainty without this information, and many examples of misdiagnosed cases exist in the older literature. Investigation of antibody deposition at various sites in adults found it to be present in biopsies of the lip, forearm, buttock and back, although in some cases the forearm biopsy was negative so this site should be avoided [75]. In young children, the back or buttock is often the most convenient site to biopsy and least distressing to the child. If possible, a biopsy of a fresh lesion (less than 24 h old) should be taken for routine histology in addition to the mandatory biopsy of uninvolved skin for direct IF.
Serum should be collected for indirect IF. Positive indirect IF can also be found from blister fluid (obtaining which is less distressing for the child and helpful in reducing the size of large blisters), and even from urine if the titres are high enough [76].
Histopathology.
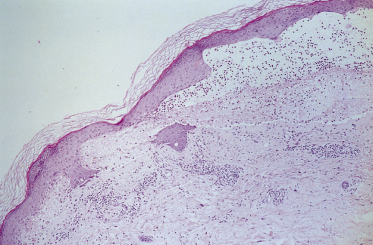
Light microscopy may be suggestive but is not diagnostic. A fresh blister (an old blister may give misleading results) may show splitting at the level of the dermoepidermal junction, but there is usually no acantholysis. A sparse dermal infiltrate is usually present, consisting predominantly of neutrophils and a few eosinophils. Collections of neutrophils in the dermal papillae, such as those occurring in DH, are sometimes seen (Fig. 89.2). One large study by Leonard et al. [57] and a subsequent comparative study between adult LAD, DH and bullous pemphigoid by Blenkinsopp et al. [77] reported subepidermal bullae in 27 out of 30 patients with LAD: only seven had eosinophils in or around the blisters, approximately one-half had papillary microabscesses, but these were multiple in one-third only. Fibrin and leucocytoclasis were found in the papillary tips in 23 out of 30 patients. Acantholysis was rarely seen in the bullae, in contrast to DH. Despite these findings, the authors comment that it is not possible to distinguish between the two diseases without IF studies. Although these studies were in patients with adult LAD, they are equally applicable to children with LAD. However, in children it is less traumatic to take a small biopsy of uninvolved skin (often best taken from a site where the child cannot observe the biopsy) to perform direct IF and ensure a positive diagnosis than to biopsy an active lesion with non-diagnostic results.
Fig. 89.2 Haematoxylin and eosin section through a fresh blister, showing a subepidermal split with an infiltrate of neutrophils and eosinophils in the dermal papillae.
Immunopathology
Direct IF
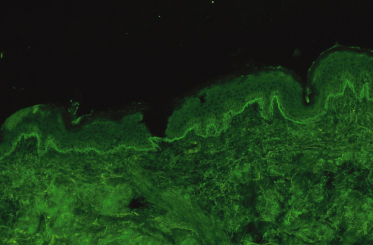
Direct IF of uninvolved skin will show a linear band with anti-IgA antibody demonstrating deposition of IgA along the BMZ (Fig. 89.3). This finding may be regarded as pathognomonic and the diagnosis cannot be made without it. False negatives do occur and the investigation may need to be repeated, particularly in the early stages of the disease. Although direct IF is a straightforward technique, laboratories where the test is performed regularly obtain a higher rate of positive results, and it may be worth repeating negative results at another laboratory. C3 and IgM may also be deposited along the BMZ in some patients [7,57,78,79]. In a group of patients IgA and IgG are found along the BMZ and these prove to be a difficult diagnostic category [7,57,78–80]. If the predominant IF is with IgA, then the authors consider such patients to have LAD provided that the only circulating antibody is IgA (see below). However, if the IF is equal with IgA and IgG, or greater with IgG, then the differential diagnosis of mixed immunobullous disease or of bullous pemphigoid should be considered [8]. A case of an adult in whom the class of immunoglobulin changed from IgG to IgA over 3 years has been reported, but no similar cases have been reported in children [81]. Clearly, this rather arbitrary division is unsatisfactory and reflects our lack of understanding of the underlying disease process.
Fig. 89.3 Direct IF (uninvolved skin) showing a bright linear band of IgA fluorescence along the dermoepidermal junction.
Indirect IF
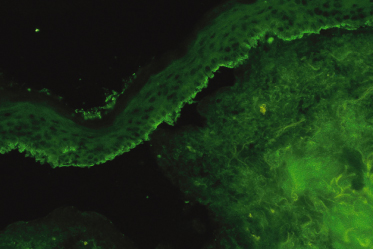
Circulating IgA antibody will be detected in up to 80% of children, which is a much higher proportion than that found in adults (less than 30%) [6,7,57,82–85]. It is usually present in a low titre (1 : 2 to 1 : 64). The sensitivity and titre using indirect IF can be increased by using salt split-skin as the substrate (Fig. 89.4), although this chiefly increases the titre in patients who are positive rather than increasing the number of positive results [86]. As with direct IF, in laboratories where the test is performed regularly, the rate of positive results is often higher, and it may be worth repeating negative results at a laboratory where the tests are performed routinely.
Fig. 89.4 Indirect IF of salt split-skin (leg), showing a linear band of IgA deposition along the roof of the split with this patient’s serum diluted 1 : 10.
The presence of additional IgG class of circulating anti-BMZ antibodies makes the diagnosis of mixed immunobullous disease.
Once patients go into remission they tend to lose their circulating antibody, but this is not sufficiently sensitive to be useful in clinical practice to monitor disease activity, unlike pemphigus vulgaris [7,87].
Distribution of Target Antigen
The antigen is found in all normal human skin and mucosae, and seems to be present in stratified squamous epithelia of most mammalian species, but not mammalian bladder [88–91]. Multiple punch biopsies taken from various sites in affected adults show antibody deposition in biopsies of the lip, forearm, buttock and back, although in some cases the forearm biopsy was negative [75]. How far this study can be applied to children is unknown but, in the authors’ experience, findings are similar. The conjunctiva, in contrast, is usually negative despite the presence of the antigen [89]. It has also been demonstrated in the amnion of placenta [88].
Ultrastructural Localization of the Antigen
Localization of antigen has been studied using both light and immunoelectron microscopy. Using light microscopy, the site of antibody deposition, and hence antigen deposition, can be studied by splitting the skin through the lamina lucida and performing both direct and indirect IF. A number of studies have been published which demonstrate heterogeneity of binding sites, with some showing epidermal and others dermal binding patterns, and a few combined [86,90,92–94].
Immunoelectron microscopy supports a dual location for the antibodies, showing a similar variation in binding sites, with either lamina lucida or sublamina densa binding or both in some patients [81,82–84,95–101]. These studies taken together suggest that there may be at least two antigens, one in the lamina lucida and one in the lamina densa associated with anchoring fibrils, and possibly dual expression in both the lamina densa and lamina lucida.
A study that compared antibody binding patterns in a cylindroma tumour and salt split-skin with patient sera, and with bullous pemphigoid and epidermolysis bullosa acquisita (EBA) sera as controls, also found two distinct patterns of binding. Those binding dermally on salt split-skin had a pattern similar to EBA sera on cylindroma tumour, suggesting that the antigen co-localizes with type VII collagen [102]. It may be that the antigens involved are of large molecular weight, are only loosely attached to surrounding structures and may traverse the whole of this region, or may be produced by the keratinocytes and secreted into the BMZ and be expressed in association with the dermal side of the lamina lucida or the lamina densa. Alternatively, it has been suggested that the antigen is secreted by dermal fibroblasts but deposited in a linear fashion on the epidermal side of the lamina lucida, and hence the antigen may be present in higher concentrations in the dermal extracts [102]. Increasing evidence is accumulating that we are dealing with a heterogeneous group of diseases with regard to the target antigens and epitopes [79,92].
Molecular Biology.
The same antigen is found in both adults and children with LAD, providing further evidence that childhood and adult disease are the same. Interestingly, the sera of all these patients bind epidermally on salt split-skin. The major antigens identified are BP180, collagen XVII and LAD285 [103–107]. The target epitopes are both the NC16a domain and the shed ectodomain of BP180 [107]. Studies have shown that several target antigens are implicated in this disease, although the response is more restricted in children [108], a feature already recognized in bullous pemphigoid. This confirms that LAD is probably more heterogeneous than originally thought with regard to the target antigens and epitopes [109–112], although clinically it appears as one disease.
Nature of the Antibody
The bound antibody found by direct IF in this disease has been shown to be of IgA class 1 in adults [113–115]. In contrast to DH, no dimeric forms are found, suggesting that the antibody in LAD is predominantly from blood- and bone marrow-derived lymphocytes, although in two cases IgA2 deposits without circulating IgA2 antibodies have been found [98,113]. J chains, which imply that the immunoglobulin is dimeric or polymeric, were demonstrated in the cutaneous deposits in five out of 15 cases of adult LAD; however, in four of these cases they could be attributed to co-existent IgM in the skin [116]. A further series also demonstrated J chains in one out of three cases [115]. These results suggest that the IgA is polymeric; however, this does not imply that IgA2 is necessarily involved as IgA1 can be polymeric. The quantity that is polymeric increases from childhood through to adulthood (0–12 years), being proportionally higher in children under 2 years of age and also in response to antigenic challenge [117,118]. Overproduction of IgA by peripheral blood lymphocytes has been studied and appears not to occur in adults with LAD, in contrast to adults with other IgA-mediated autoimmune diseases, and the presence of circulating IgA antibody found in LAD is a result of specific antigenic stimulation. However, titres of IgA antigliadin antibodies have been found to be higher than titres of IgG antibodies, suggesting that these patients may preferentially mount an IgA response [51,119,120]. Although other workers have not found an increased IgA response to dietary antigens [51,121], pathogenicity of the IgA antibody has been demonstrated in vitro using normal human skin cultures, by demonstrating that binding to the BMZ occurs and produces separation at the dermoepidermal junction [122].
Major Histocompatibility Complex Associations
A study of over 60 patients which looked at class I and II MHC associations found an increased incidence of HLA-B8, HLA-DR3 and HLA-DQw2 that was more marked in children than in adults [34]. This difference can largely be explained by the fact that five children were homozygous for B8, DR3 and DQw2. The children homozygous for these alleles presented with the disease earlier than the group as a whole. Almost all patients possessed either DR2 or DR3 (92% CBDC and 80% adult LAD), with 71% of children and 46% of adults possessing the DR3 allele. A strong association with Cw7 was also found (77% of adults and 75% of children); furthermore, there was an almost total absence of HLA-DR1 and HLA-DR4 alleles carrying the ‘rheumatoid motif’ in these patients. Other groups have also found an increased incidence of HLA-B8 in children and adults, although the incidence was less in the adults [6,7,57,123–125]. An association with DR3 has also been shown in adults [126]. The differences between the adults and children in these studies do not refute the hypothesis that both groups are part of the same disease, and perhaps possession of the B8 and DR3 alleles facilitates presentation of the disease at a younger age.
Linear immunoglobulin A disease in childhood was found to be associated with possession of the unusual tumour necrosis factor type 2 (TNF-2) allele in 19 out of 26 children, and possession of the rare TNF-2 allele was associated with a worse prognosis and longer disease duration (5.3 versus 3.0 years). TNF-2 appears to be in linkage disequilibrium with the DR3 allele, and patients who were homozygous for TNF-2 had a lower mean age of onset, as might be expected [34].
Clinical Features.
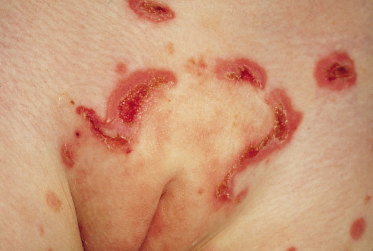
The clinical presentation of childhood LAD/CBDC is so characteristic that it was recognized as an entity in the 1890s. The classic presentation is in a preschool child with tense blisters, some arising from urticated plaques and some from normal skin. The disease usually presents abruptly and may be accompanied by marked systemic illness, fever and anorexia, although in some patients the onset is more insidious, with a pruritic rash over a number of weeks but without the long prodromal pruritus sometimes seen with bullous pemphigoid [18,20,23]. The lesions are often polycyclic or annular with blistering at the edges; this sign was given the rather overly romantic name of the ‘string of pearls’ but the morphology of the rash usually changes with time. The blisters are typically small (1–3 mm) and tense, often arranged in rosettes on an erythematous background around the edge of an annular lesion (Figs 89.5, 89.6). Large bullae, often haemorrhagic, are sometimes seen, and milia may rarely follow. The lesions characteristically affect the perineal area with spread onto the trunk and thighs, and frequently the limbs, hands and feet (Fig. 89.7). Facial involvement, particularly of the perioral area, is common; however, any site on the body may be affected.
Fig. 89.5 Small, tense blisters arranged in rosettes on an erythematous background – the ‘string of pearls’ sign.
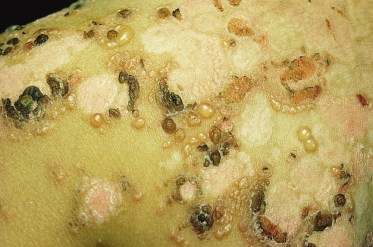
Fig. 89.6 Blisters arranged in rosettes on an erythematous background around the edges of annular and polycyclic lesions.
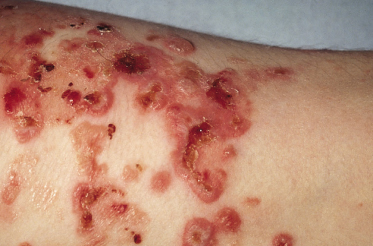
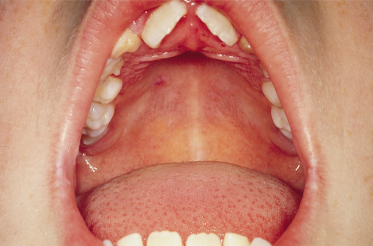
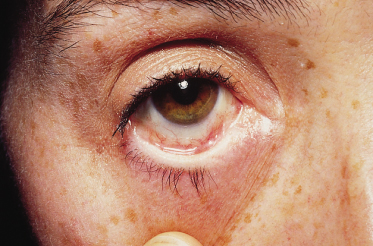
Mucosal involvement, with a few exceptions, was not recognized until 1984 [16,20,22,23] [1,2,4,5]. Mucosal involvement is chiefly oral, but can be ocular, nasal or genital, occasionally with scarring [7,22,127,128,129] [5–9]. In a series of 24 children with childhood LAD, mucosal involvement was found in 91%, with erosions and discomfort being the usual findings, although, occasionally, intact blisters were found (Fig. 89.8); 65% had ocular involvement, an observation similar to other series. Ocular erosions may involve the conjunctiva and lead to scarring. In one series, scarring of the conjunctiva was estimated to occur in 43% of patients reporting eye symptoms. The children in whom ocular involvement led to blindness are best regarded as having MMP (see below) (Fig. 89.9) [7,22,127,130]. Oral scarring has also been described [128].
The mean age of onset of the disease is under 5 years with a range of 1–11 years. The initial attack is sudden and more severe than following recurrences.
A common presentation in children is with genital blisters and erosions, which has led to a misdiagnosis of herpes simplex and, more seriously, we are aware of two cases in which the possibility of sexual abuse was considered. Hypopigmentation and hyperpigmentation may be prominent, especially in those patients with deeply pigmented skin. The severity of the disease is very variable, from just a few small blisters to extensive areas of the body being involved and severe mucosal erosions. It is likely that some mild cases never reach the attention of dermatologists and go undiagnosed.
Prognosis.
In approximately 65% of children, the disease goes into remission defined as ‘no clinically apparent disease with the patient receiving no treatment’ [7]. The mean disease duration is 3–5 years, after which spontaneous remission occurs in the majority of patients [87]. In those who fail to go into remission, the disease may persist into adult life, and there are reports of patients with disease persisting for 28 and 33 years [87,95]. It is possible that some very transient cases are never diagnosed. The authors have found no correlation between severity and duration of disease, and have found no factors, apart from MHC typing, which have proved useful predictors of the course or outcome in paediatric cases, including the site of involvement, age of onset, sex and response to treatment, although earlier reports have suggested that ocular damage is more likely with long-standing disease and an absence of HLA-B8 [87]. MHC typing in a large series has shown that possession of the rare TNF-2 allele is associated with longer disease duration (5.3 versus 3.0 years) [34]. Other MHC associations do not appear to affect prognosis.
There are a number of reports of adult LAD occurring in association with lymphoproliferative malignancies; in particular, there are four reports of LAD in association with Hodgkin’s disease, one report in association with gastric lymphoma and a report of two cases, one with chronic lymphatic leukaemia and the other with a plasmacytoma [125,131–134,135]. Finally, there is one report of myeloma following LAD that had gone into remission, and a single case occurring with polycythaemia rubra vera [125]. Only two comparable cases have been reported in children, which may well reflect their young age rather than a true difference from the adults, and it is not known whether these children will be at increased risk of developing lymphoproliferative malignancies in later life [7].
Godfrey et al. [41] looked at a large series of 70 patients with adult LAD for associations with malignancy including previously reported patients. They found three cases of B-cell lymphoma, a significant increase compared with the expected incidence from the National Cancer Registry. It is interesting to note the similarity to DH where an association with lymphoma is widely accepted [136]. No association with solid tumours was found, although a total of nine malignancies was recorded, which almost exactly matched the expected number among age- and sex-matched control subjects. No particular tumour was involved; all nine tumours were different. Despite this study, a number of anecdotal reports of tumours in association with adult LAD have been published, including thyroid carcinoma occurring simultaneously, a uterine adenocarcinoma preceding the onset of adult LAD, and a carcinoma of the bladder which, interestingly, showed no antigen expression [87,137,138]. No such disease association has been reported in children but this does not mean it does not occur, and only long-term follow-up of affected children will resolve this question.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree