Follicular Hyperkeratinization and Comedogenesis
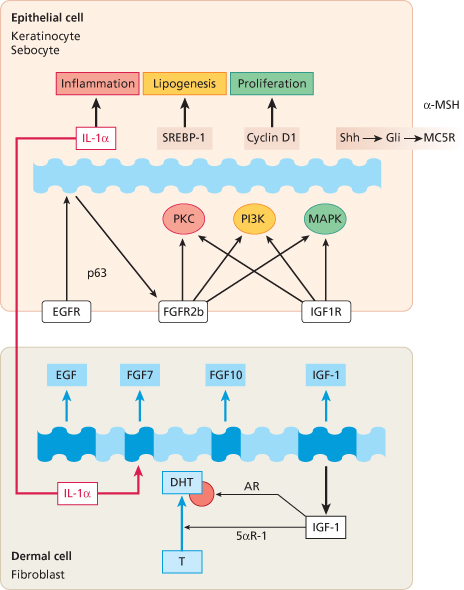
One of the first steps in acne pathogenesis is the formation of the microcomedo, which begins in the keratinized epithelium of the upper portion of the follicle, the infundibulum. Corneocytes, which are normally shed into the lumen of the follicle, are retained and accumulate. Two mechanisms are involved in comedo formation: exaggerated cellular proliferation and increased intercorneocyte cohesiveness [91]. Proliferation of basal keratinocytes and sebocytes is regulated by epidermal growth factor receptor (EGFR). IGF1R expressed on basal and suprabasal cells co-ordinates cellular proliferation and differentiation, whereas fibroblast growth factor receptor-2b (FGFR2b), predominantly expressed on suprabasal keratinocytes and sebocytes, primarily regulates later steps of cellular differentiation [51]. Fibroblasts of the adjacent dermis of sebaceous glands secrete IGF-1 and fibroblast growth factor (FGF) 7 and FGF10. Especially under increased androgen stimulation, fibroblasts synthesize and secrete FGFs, which bind to FGFR2b expressed on keratinocytes and sebocytes.
This dermal-epithelial interaction is of crucial importance for comedogenesis, sebaceous gland proliferation and lipogenesis [92,93]. The FGFR2 gain-of-function mutations in Apert’s syndrome and unilateral acneiform naevus pointed the way to androgen-dependent dermal-epithelial FGFR2 signalling in acne [49–51]. There is also epidermal-dermal interaction regulating proliferation and differentiation. Keratinocyte-derived interleukin-1α (IL-1α) stimulates dermal fibroblasts to secrete FGF7, which subsequently activates FGFR2b-mediated keratinocyte proliferation in a double paracrine pathway [94]. IL-1α has been detected in increased concentration during the early steps of comedo formation [46,48]. The importance of FGFR2b signal transduction for sebaceous gland homeostasis is supported by the observation that severe sebaceous gland atrophy results after postnatal deletion of FGFR2b [93]. It has recently been suggested that all known anti-acne agents are able to attenuate increased FGFR2b signalling [95]. Insulin and IGF-1 interact with androgen-dependent FGFR2b signalling and link androgen- and FGF-mediated signal transduction pathways which are both important in acne pathogenesis (Fig. 79.2). FGFR2b acts upstream of several matrix metalloproteinases, sonic hedgehog and melanocortin receptors, which contribute to acne pathogenesis [96–99].
Fig. 79.2 Dermal-epithelial growth factor signal transduction of tyrosin kinase receptors in acne. T, testosterone; DHT, dihydrotestosterone; AR, androgen receptor; IGF-1, insulin-like growth factor-1; 5αR-1, 5α-reductase type 1; EGF, epidermal growth factor; FGF, fibroblast growth factor; IL-1α, interleukin-1α; p63, transcription factor p63; EGFR, EGF receptor; FGFR2b, FGF receptor 2b; IGF1R, IGF-1 receptor; PKC, protein kinase C; PI3K, phosphoinositide-3-kinase; MAPK, mitogen-activated protein kinase; SREBP-1, sterol response element binding protein-1; Shh, sonic hedgehog; Gli, transcription factor Gli; MC5R, melanocortin-5 receptor; α-MSH, α-melanocyte stimulating hormone.
In the underlying follicular epithelium of the comedo, keratohyaline granules are increased in size and number, whereas lamellar granules and tonofilaments are decreased. The sebaceous lobule undergoes regression due to the expansion of the comedo. With further expansion of the comedo, sebum-surrounded keratinocytes create concentric lamellar concretions. Rupture of the comedo wall with extrusion of immunogenic content results in inflammation. Prior to follicular hyperkeratinization and comedo formation, increased IL-1 activity and CD4+ T-cell infiltration has been observed in acne-prone skin areas [100]. When neutrophils predominate, pustular lesions are formed. A mixed infiltrate consisting of T-helper cells, foreign body type giant cells and neutrophils may lead to inflamed pustules, nodules and cysts. The type of infiltrate determines the degree of scarring, which is strongly associated with a delayed specific inflammatory response [101].
The Role of Propionibacterium Acnes in Inflammation
Propionibacterium acnes, a Gram-positive non-motile, rod-shaped anaerobic bacterium, plays a critical role in the progression of inflammation in acne vulgaris [102,103]. P. acnes and Staphylococcus epidermidis are members of the resident bacterial flora and mostly reside in the pilosebaceous follicle. Staphylococcus epidermidis is found in the mid-infundibulum while P. acnes predominates in deeper portions of the follicle in much higher numbers in acne patients than in normal controls. Both bacterial species secrete lipases and hydrolyse triglycerides to free fatty acids. P. acnes synthesizes porphyrins, primarily coproporphyrin III, so that colonized follicles fluoresce in Woods light, the basis of photodynamic therapy of acne with blue light. The complete genome sequence of P. acnes has been characterized [104].
Although increased numbers of P. acnes have been found in acne patients, their numbers do not correlate with clinical severity [105]. It is conceivable that AR-, IGF1R- and FGFR2b-mediated hyperkeratinization of the infrainfundibular epithelium modifies the microenvironment for hypercolonization of P. acnes. Although the contribution of P. acnes in the initiation of comedogenesis is still controversial, its role in inflammation is well established. P. acnes produces a number of extracellular enzymes and metabolites that can directly damage host tissue. It stimulates production of proinflammatory cytokines, including IL-1β, IL-8, IL-12 and tumour necrosis factor-α (TNF-α). The increase in IL-8, in particular, results in neutrophil recruitment, the release of lysosomal enzymes, and subsequent disruption of the follicular epithelium. The P. acnes-induced cytokine productions are mediated by toll-like receptor-2 (TLR2) [106–108]. Sapienic acid, a fatty acid in sebum, has innate antimicrobial activity and is upregulated by activation of TLR2 by skin bacteria [109,110].
Matrix metalloproteinases (MMPs) are important enzymes implicated in the remodelling of extracellular matrix in both physiological and pathological conditions. P. acnes increased the expression of pro-MMP-2 through TNF-α in human dermal fibroblasts, a process which could be inhibited by doxycycline [111]. FGFR2b signalling pathways are also involved in downstream expression of MMPs [112]. Growth and expansion of P. acnes are controlled by antimicrobial peptides and antimicrobial lipids derived from sebaceous glands. Upregulation of human β-defensin-1 and -2 has been observed in acne lesions [113]. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill P. acnes [114]. Reduction in P. acnes numbers by antimicrobial agents correlates with the clinical improvement of acne. However, P. acnes strains with antibiotic resistance were identified from acne patients treated for longer than 3 months [115,116]. Biofilm formation of P. acnes increases resistance against antimicrobial agents [117]. IgG-coated bacteria were found in comedones of acne patients, pointing to a plasma-derived humoral immune response [118]. The contribution of P. acnes to inflammation in acne is supported by the observation that vaccination with inactivated P. acnes suppressed inflammation and the production of IL-8 in human sebocytes [119,120].
Pathology.
The clinical findings in acne can be divided into primary non-inflammatory lesions, secondary inflammatory lesions, and postinflammatory lesions.
Primary Non-Inflammatory Lesions
The microcomedo is the first detectable morphological change of the sebaceous follicle seen only in the microscope, appearing as a keratinaceous plug in the infrainfundibulum. The granular layer at this stage is prominent. Proliferation and retention hyperkeratosis create a tightly compressed layer of 40–80 corneocytes surrounding vellus hairs admixed with sebum and an increased number of bacteria [42,91]. The plug leads to a distal expansion of the follicle resembling a balloon. In closed comedones (whiteheads), the degree of follicular distension is increased, and a compact cystic structure forms which contains eosinophilic, keratinaceous debris, vellus hairs and numerous bacteria. When the infrainfundibulum is filled with coneocytes, while the acroinfundibulum remains tightly closed, skin-coloured whitish papules are seen. Open comedones (blackheads) develop when the acroinfundibular corneocyte plug is further enlarged. The melanin content is responsible for the black colour. Long-standing comedones can accumulate up to 15 vellus hairs, which may elicit foreign body reactions when extruded into the dermis. The sebaceous glands are typically atrophic or absent and a mild perivascular mononuclear cell infiltrate surrounds the expanding follicle.
Secondary Inflammatory Lesions
Early open comedones already contain increased amounts of IL-1α and T-cells [46]. Any primary lesion can become inflamed. Overgrowth of P. acnes with secretion of inflammatory mediators, in particular IL-8, will attract neutrophils, resulting in the formation of papules and pustules. As the follicular epithelium distends, the immunogenic cystic contents may rupture into the dermis, inducing further accumulation of neutrophils and finally leading to foreign body granulomatous inflammation. Indurated nodules are persistent deep lesions that result from long-lasting dermal inflammation and are often called acne cysts, although they do not present a cystic structure. Abscesses, especially in acne conglobata, develop when neighbouring papules or pustules coalesce. Indurated inflammatory nodules can drain blood, pus and sebum. Draining sinuses are typically seen in acne conglobata and acne fulminans and are found in the nasolabial region, the bridge of the nose, the mandibular region and the neck. Linear fluctuant abscesses up to 10 cm in length contain numerous sinus tracts or fistulas connected to the skin surface. With pressure, they discharge foul-smelling secretions appearing at several orifices simultaneously.
Postinflammatory Lesions
These result from more inflammatory acne. Fistulated comedones, most commonly double comedones, are usually found on the neck and back. Epidermoid cysts are seen in the retroauricular region and along the eyebrows, are 1–5 cm in diameter, elevated and present a prominent central pore. Typical acne scars include ice-pick or punched-out lesions, nodular scarring, keloid scars, predominantly seen on the chest and back, and large atrophic scars.
Clinical Features.
Acne can be divided into three types: comedonal acne, papulopustular acne and acne conglobata. The first two types are often called acne vulgaris. In acne conglobata, a more intense inflammatory reaction occurs which leads to more severe scarring and postinflammatory lesions.
Comedonal Acne
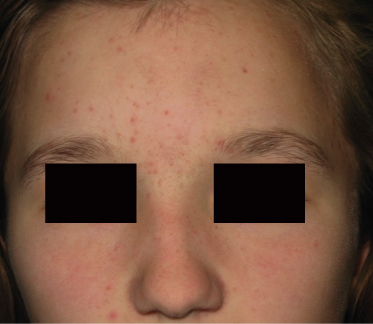
In comedonal acne (Fig. 79.3), open and closed comedones predominate and often appear during prepuberty on the nose, midface and forehead. Increased serum levels of DHEAS during adrenarche have been correlated with increased sebum production and the appearance of comedonal acne [64,65].
Papulopustular Acne
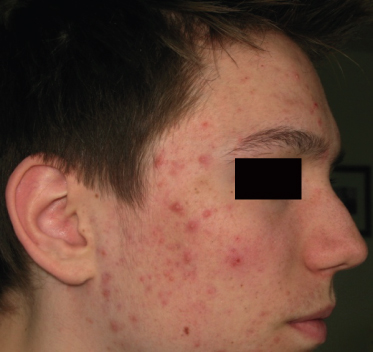
Inflammatory acne originates from comedones which become inflamed and transform to papules and pustules (Fig. 79.4). The face, chest and back may be involved. Few comedones may also be present. Some lesions become markedly inflamed, indurated and tender and form inflammatory nodules. Scarring usually occurs. Papulopustular acne may persist for many years.
Acne Conglobata
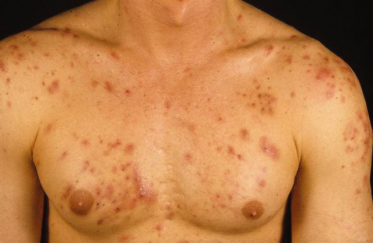
This most severe form of acne affects men more often than women. The appearance of nodulocystic acne is associated with severe dermal tissue changes. Nodules and cysts frequently coalesce to form inflammatory plaques that can include sinus tracts. Abscesses, draining sinuses, fistulated comedones, keloidal and atrophic scars develop. Usually the face, chest and back are affected, but the disease might involve the arms, abdomen, buttocks and scalp (Fig. 79.5). The course is chronic in most patients with acute exacerbations. Acne conglobata may be part of the follicular occlusion tetrad, along with dissecting cellulitis of the scalp, acne inversa (hidradenitis suppurativa) and pilonidal cysts.
Prognosis.
Acne often becomes a chronic disease and may not be just a self-limiting disorder of teenagers. Acne can persist into adulthood in as many as 50% of individuals [121]. Early and prolonged treatment of acne is essential for the prevention of scarring. Persistent erythema and postinflammatory hyperpigmentation often remain after successful treatment of inflammatory lesions. Pitted or nodular hypertrophic scars are sequelae of acne conglobata. Hypopigmented anetoderma-like lesions may be seen on the upper trunk. Some patients with acne conglobata develop painful, pruritic disabling keloids, especially on the chest, shoulders and upper back.
Treatment.
The Global Alliance to Improve Outcomes in Acne has published recommendations for the management of acne [121] and established a website (www.acneglobalalliance.org). Table 79.1 presents an acne treatment algorithm for varying degrees of acne severity [122]. Acne can be treated topically as well as systemically and with combinations of both topical and systemic agents.
Table 79.1 Global Alliance acne treatment algorithm according to Gollnick et al. 2003 [122]
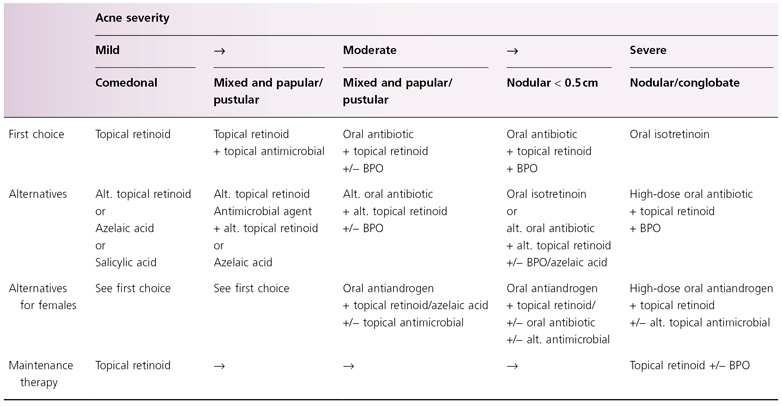
Alt, alternatives; BPO, benzoyl peroxide.
Topical Therapy
Topical Retinoids.
The first topical comedolytic agent used for the treatment of acne was tretinoin. At least five topical retinoids are available for acne treatment: tretinoin (all-trans-retinoic acid) 0.01–0.1% cream or gel, isotretinoin (13-cis-retinoic acid) 0.1% gel, adapalene (an aromatic naphthoic acid derivative) 0.1–0.3% gel, tazarotene (a synthetic acetylenic retinoid) 0.05–0.1% gel, and motretinide (second-generation monaromatic retinoid) 0.1% cream. Tretinoin normalizes follicular hyperkeratinization, has comedolytic effects and prevents the formation of new comedones. Tretinoin is effective in the treatment of comedonal acne and mild acne vulgaris. Its most common side-effect is local irritation, which clinically appears as erythema, dryness and scaling. Patients should be advised to increase the amount and frequency of application stepwise. In most cases, topical retinoids are used once daily in the evening. In comparison with other topical retinoids, adapalene has the lowest potential for skin irritation. Topical retinoids should be avoided during pregnancy and breastfeeding due to their potential teratogenic effects. However, an interruption of pregnancy is not recommended for those who have used topical retinoid treatments during pregnancy [123]. Retinoids are available in combination with antibiotics or benzoyl peroxide [124–126]. The combination of a topical retinoid and an antimicrobial agent remains the preferred approach for almost all patients with acne, as this combination attacks three of the four pathogenetic factors in acne: abnormal follicular keratinization, hypercolonization of P. acnes, and inflammation [121,122,127].
Benzoyl Peroxide.
Benzoyl peroxide is a potent bacteriocidal agent that rapidly reduces the levels of P. acnes within the follicle. After application, free oxygen radicals are generated which exert an antimicrobial effect. In contrast to topical antibiotics, bacterial resistance to benzyol peroxide has not been observed. Various formulations including soaps, washes, gels and lotions in concentrations of 2.25% to 10% are available. Benzoyl peroxide is very effective in inflammatory acne. Patients should be advised that whitening of clothing and bedding or other coloured fabrics can occur due to its bleaching activity. Rarely, allergic contact dermatitis can develop. Benzoyl peroxide can be combined with topical retinoid treatment. Fixed-dose combination products of benzyol peroxide with clindamycin or adapalene are currently available [128,129].
Azelaic Acid.
Azelaic acid, a saturated dicarboxylic acid, is used as a helpful adjunct in treating comedonal and inflammatory acne as a 15% gel or 20% cream [130,131]. It inhibits the growth of P. acnes and normalizes altered follicular keratinization. Azelaic acid is applied twice daily. Due to its minor irritation potential in comparison with topical retinoids, its use in patients with atopic skin diathesis may be of advantage. In addition, it may reduce postinflammatory hyperpigmentation.
Topical Antibiotics.
Four groups of antibiotics are available for topical treatment in concentrations ranging from 1% to 4%: erythromycin, clindamycin, tetracycline and nadifloxacin [132]. They have bacteriostatic effects, except nadifloxacin which is bacteriocidal, and inhibit Gram-positive bacteria. Erythromycin (2%, 4%) and clindamycin (1%) are commonly prescribed for inflammatory acne. Monotherapy with a topical antibiotic can result in the development of resistant strains of P. acnes. A significant proportion of patients with acne are colonized with resistant P. acnes even before treatment is initiated [133]. To reduce the problems with resistance, topical and systemic antibiotics should not be combined and antibiotics should be used together with benzoyl peroxide or retinoids [121]. Clindamycin and nadifloxacin can be life-saving drugs for other indications and should therefore be reserved for systemic bacterial infections.
Salicylic Acid.
Salicylic acid was formerly a mainstay of acne therapy due to its keratolytic action and is still available in over-the-counter preparations, but it is not as effective as the agents described above.
Dapsone.
A 5% topical gel has recently become available which seems clinically effective in reduction of inflammatory lesions [134,135]. The topical preparation is not associated with the adverse effects of systemic dapsone treatment.
Oral Treatments
Systemic treatment is required for widespread papulopustular acne and acne conglobata. Oral antibiotics, oral isotretinoin and antiandrogenic therapy may be used.
Tetracyclines.
Oral minocycline and doxycycline are usually prescribed for moderate to severe inflammatory acne unresponsive to topical treatment [136,137]. Systemic antibiotics should not be given as monotherapy, and should be combined with topical benzoyl peroxide or topical retinoid in order to avoid the development of resistant bacterial strains. A typical course of tetracycline derivative might include minocycline given over 3–4 months. Therapy is initiated with a dose of 50–100 mg b.i.d. and reduced to 50 mg daily for maintenance therapy. Extended-release forms of minocycline are available and can be given at 45–135 mg as a single daily dose, depending on the patient’s bodyweight. Tetracyclines reduce P. acnes and inhibit the expression and activity of MMPs involved in dermal tissue remodelling [95]. Tetracylines cannot be combined with oral isotretinoin because both drugs have the potential for raising intracranial pressure and, when combined, increase the risk of pseudo-tumour cerebri. Doxycycline is given initially in a daily dose of 100–200 mg. It is associated with an increased incidence of photosensitivity and gastrointestinal side-effects when compared with minocycline. Tetracyclines should be avoided in children under the age of 8 years because of side-effects related to dentition and bone formation.
Macrolides.
Oral erythromycin, clarithromycin, roxithromycin and azithromycin are not commonly used in acne. However, they can be used to treat acne in pregnancy. They are prescribed in doses ranging from 150 to 250 mg b.i.d. for several weeks.
Oral Isotretinoin.
Systemic isotretinoin (13-cis-retinoic acid) is the most effective treatment for severe and persistent acne [138]. Isotretinoin modifies all four major pathogenetic factors of acne. It reduces comedogenesis, leads to involution of sebaceous glands (90%), reduces sebum production and hypercolonization of P. acnes, and diminishes inflammatory reactions [139,140]. Isotretinoin induces apoptosis and cell cycle arrest in sebocytes [141]. It is most effective for the treatment of inflammatory acne and should be initiated early to reduce the risk of scar formation [142]. The usual dose is 0.2–0.5 mg/kg daily in a single dose with a fatty meal to improve intestinal absorption. Rarely, higher daily doses of 1.0 mg/kg are required. The usual treatment period is 3–5 months. Good responses in moderate acne have been reported with low-dose treatment (20 mg/day) [143]. Others have suggested that a total cumulative dose of >100–120 mg/kg may reduce the potential for relapse following completion of isotretinoin. In general, isotretinoin therapy is not recommended in children below the age of 12 years. Low-dose isotretinoin has been successfully administered in combination with topical tretinoin [144]. Low-dose treatment is not recommended for severe acne papulopustulosa or acne conglobata as response to treatment is delayed and associated with a higher rate of relapse [122,145]. Repeat courses of isotretinoin may be necessary.
Before starting therapy, the following laboratory parameters should be obtained: CBC, liver function tests, serum levels of cholesterol, triglycerides, creatinine and bilirubin. Transaminases and lipid tests should be repeated monthly. Adverse effects are dose dependent and include cheilitis, dry eyes, dry nose with nosebleeds, increased sensitivity to sunlight exposure and xerosis in most patients. Patients should be instructed to regularly use lip balm, skin moisturizing ointments and artificial tears if necessary. More serious but less common adverse effects include musculoskeletal complaints, pseudo-tumour cerebri, hypertriglyceridaemia and hypercholesterolaemia, and hyperostosis at sites of tendon insertion. Contact lens users should switch to glasses to avoid eye irritation. The patient should be advised that night vision may be impaired, especially when driving at night. Before refractive laser eye surgery is performed, isotretinoin treatment should be terminated for at least 6 months.
Isotretinoin is a potent teratogen and can cause isotretinoin embryopathy syndrome with facial malformations, cardiac and CNS defects, and mental retardation. The patient and parents of minors must be extensively counselled and be asked to sign an informed consent. Two forms of contraception should be used for the entire treatment period and 3 months thereafter. Female adolescents must use oral contraceptives at least 1 month prior to starting oral isotretinoin. Two negative pregnancy tests are required. The donation of blood and the administration of the drug to other persons are prohibited [142]. The prescribing physician should consider governmental regulatory instructions for isotretinoin use which vary from country to country.
Isotretinoin has been associated with depression, suicidal ideation and even suicide. In the ongoing controversy about isotretinoin’s psychiatric adverse effects, dermatologists point out that concrete scientific data limit any conclusion about a causal relationship between isotretinoin and psychiatric adverse events [146] and report an alleviation of depressive symptoms due to improvements in acne grade [147]. On the other hand, psychiatrists point to the association between isotretinoin use and psychopathology and conclude that the multiformity of reported psychiatric adverse events is probably associated with the multiplicity of isotretinoin’s effects on various neurotransmitter systems and with the various types of vulnerability of the exposed individuals [148]. The WHO registered 47 suicides, 67 suicide attempts and 56 suicide ideations associated with oral isotretinoin from 1982 to 1998. The total adverse drug reactions (including suicide, suicide attempt and suicide ideation) per million estimated patient exposures were 28.3 [148]. Therefore, clinicians should inform patients and parents about the possibility of rare psychiatric adverse effects, should take a family history on psychiatric disorders and should be on the alert for potential psychiatric side-effects following treatment with isotretinoin, especially in vulnerable persons [148].
Recent attention has also been paid to the association between isotretinoin and inflammatory bowel disease (IBD). While the specific nature of the association between isotretinoin and IBD has not yet been fully elucidated, it is prudent to counsel patients and families about the potential risks of IBD in those receiving isotretinoin.
Antiandrogens.
Drug combinations of cyproterone acetate (2 mg)/ethinyl oestradiol (35 µg), drospirenone (3 mg)/ethinyl oestradiol (30 µg) and desogestrel (25 µg)/ethinyl oestradiol (40 µg) for 1 week followed by desogestrel (125 µg)/ethinyl oestradiol (30 µg) for 2 weeks showed the strongest antiandrogenic activity on acne. Treatment with antiandrogens should only be considered if none of the contraindications exists. Antiandrogen treatment should be limited to female patients with additional signs of peripheral hyperandrogenism or hyperandrogenaemia. In addition, women with late-onset or recalcitrant acne who also desire contraception can be treated with antiandrogens, as can those being treated with systemic isotretinoin. Antiandrogen treatment is not appropriate as primary monotherapy for non-inflammatory and mild inflammatory acne [44]. It should be noted that oral contraceptives, especially gestagens with androgenic activity, impair insulin resistance [149,150], which is an unfavourable contribution to the pathogenesis of acne and, as with other contraceptive agents, have also been associated with an increased risk of venous thromboembolism [8,39].
Insulin-Sensitizing Agents.
Insulin resistance and hyperinsulinaemia have been observed in prepubertal girls with premature adrenarche. Premature pubarche shares many clinical characteristics with PCOS [62]. Girls with low birthweight and precocious pubarche are at risk for early onset of puberty and menses and further progress to anovulation, hyperinsulinaemic hyperandrogenism, PCOS and acne [151]. It appears that the IGF-1 axis in small-for-gestational age/low birthweight newborns later drifts to higher IGF-1 levels which can promote the development of precocious pubarche and PCOS. PCOS is associated with increased serum levels of IGF-1, DHEAS, hyperinsulinaemia, insulin resistance, acne and hirsutism [152]. Metformin is the therapy of choice for PCOS and has been demonstrated to reduce serum androgen levels and gonadotropins with an improvement of acne and hirsutism, menstrual cycle, ovulation and fertility [153,154]. Metformin treatment prevented the onset of early puberty in girls with precocious pubarche and significantly decreased serum levels of IGF-1, fasting insulin, DHEAS and testosterone [155]. Thus, the metformin-mediated ‘counter-regulatory’ action on increased insulin/IGF-1 signalling might be used in the treatment of acne in the near future [156].
Future Treatment Options
New developments and future trends include low-dose long-term isotretinoin regimens, new isotretinoin formulations (micronized isotretinoin), isotretinoin metabolites, combination treatments to reduce toxicity, insulin-sensitizing agents, 5α-reductase type 1 inhibitors, androgen receptor antisense oligonucleotides, lipoxygenase inhibitors, topical FGFR2b and IGF1R tyrosine kinase inhibitors, PI3K inhibitors and ectopeptidase inhibitors [51,79,95,157,158].
Dietary Intervention
The current status of the relationship of diet and acne is under debate. The 2007 recommendations of the American Academy of Dermatology suggested that caloric restriction has no benefit in the treatment of acne and there is insufficient evidence to link the consumption of certain ‘food enemies’ to acne [159]. However, recent evidence shows that insulin resistance, hyperinsulinaemia and increased serum IGF-1 serum levels are involved in the pathogenesis of acne [156]. An improvement of acne and biochemical parameters has been observed during a 12-week randomized controlled study with a low glycaemic load diet [160,161]. Improvement of acne has been reported during a carbohydrate-restricted diet from South Beach Florida [162,163]. An epidemiological dose-dependent correlation between milk consumption and acne in teenage boys and girls has recently been recognized [5,6]. High intake of milk in children switches the somatotopic axis (increase in GH, IGF-1 and insulin) to higher levels and induces insulin resistance [164,165]. These observations make it reasonable to recommend an empirical trial of dietary restriction of milk intake and hyperglycaemic carbohydrates to improve acne [8,156].
Restriction of Smoking
Data on the role of smoking in acne are still controversial. Increased, unchanged as well as decreased prevalence of acne has been reported in smokers [166–170]. It should be noted that smoking leads to insulin resistance [171,172]. The controversy might be clarified when pre-existing insulin resistance like transient insulin of puberty or insulin resistance due to increased AR signalling and nutritional causes are discriminated. In women with PCOS, smoking substantially aggravated insulin resistance [173]. Smoking may be a risk factor for acne in individuals who have a genetic disposition for the development of insulin resistance or are exposed to environmental risk factors like high glycaemic food and milk consumption. Patients with acne should refrain from smoking in order to avoid further deterioration of insulin sensitivity.
Physical Approaches
Cosmetic improvement can be obtained by the mechanical expression of comedones. Excision of cystic lesions and punch elevation of depressed scars may be helpful. Improvement of scars can be obtained with resurfacing lasers in the hands of experienced physicians. Keloid acne scars can be treated with injection of intralesional triamcinolone acetonide.
Radiation with UV-A or UV-B is not recommended in acne. Blue light therapy improves acne and is used to diminish the hypercolonization of porphyrin-synthesizing P. acnes [174]. Photodynamic therapy is still in the experimental phase and complicated with adverse effects [175].
References
1 Rademaker M, Garioch JJ, Simpson NB. Acne in schoolchildren: no longer a concern for dermatoloigsts. BMJ 1989;298:1217–19.
2 Kilkenny M, Merlin K, Plunkett A et al. The prevalence of common skin conditions in Australian school students, III: acne vulgaris. Br J Dermatol 1998;139:840–5.
3 Lello J, Pearl A, Arroll B et al. Prevalence of acne vulgaris in Auckland senior high school students. N Z Med J 1995;108:287–9.
4 Cordain L, Lindeberg S, Hurtado M et al. Acne vulgaris. A disease of Western civilization. Arch Dermatol 2002;138:1584–90.
5 Adebamowo CA, Spiegelman D, Berkey CS et al. Milk consumption and acne in adolescent girls. Dermatol Online J 2006;12:1–12.
6 Adebamowo CA, Spiegelman D, Berkey CS et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol 2008;58:787–93.
7 Danby FW. Acne and milk, the diet myth, and beyond. J Am Acad Dermatol 2005;52:360–2.
8 Melnik B. Milk consumption: aggravating factor of acne and promoter of chronic diseases of Western societies. J Dtsch Dermatol Ges 2009;7:364–70.
9 Smith R, Mann N, Braue A et al. The effect of a high protein, low glycemic load diet versus a conventional, high glycemic load diet on biochemical parameters associated with acne vulgaris. J Am Acad Dermatol 2007;57:247–56.
10 Smith R, Mann N, Mäkeläinen H et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res 2008;52:718–26.
11 Kerr JM, Congeni JA. Anabolic–androgenic steroids: use and abuse in pediatric patients. Pediatr Clin North Am 2007;54:771–85.
12 Johnston LD, O’Malley PM, Bachman JG et al. Monitoring the future national survey results on drug use, 1975–2005. In: College Students and Adults Ages 19–45, vol. II. NIH Publication No. 06–5884. Bethesda, MD: National Institute on Drug Abuse, 2006.
13 Melnik B, Jansen T, Grabbe S. Abuse of anabolic–androgenic steroids and bodybuilding acne: an underestimated health problem. J Dtsch Dermatol Ges 2007;5:110–17.
14 Melnik BC. Androgen abuse in the community. Curr Opin Endocrin Diabetes Obesity 2009;16:218–23.
15 Bataille V, Snieder H, MacGregor AJ et al. The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol 2002;119:1317–22.
16 Ballanger F, Baudry P, N’Guyen JM et al. Heredity: a prognostic factor for acne. Dermatology 2006;212:145–9.
17 Evans DM, Kirk KM, Nyholt DR et al. Teenage acne is influenced by genetic factors. Br J Dermatol 2005;152:565–95.
18 Hsing AW, Gao YT, Wu G et al. Polymorphic CAG and GGN repeat length in the androgen receptor gene and prostate cancer risk: a population-based case control study in China. Cancer Res 2000;1518:5111–16.
19 Beilin J, Ball EM, Favaloro JM, Zajac JD. Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J Mol Endocrinol 2000;25:85–96.
20 Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res 1994;11:3181–6.
21 Zeegers MP, Kiemeney LA, Nieder AM et al. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev 2004;13:1765–71.
22 Sutcliffe S, Giovannucci E, Isaacs WB et al. Acne and risk of prostate cancer. Int J Cancer 2007;121:2688–92.
23 Legro RS, Shahbahrrami B, Lobo RA et al. Size polymorphisms of the androgen receptor among female Hispanics and correlation with androgenic characteristics. Obstet Gynecol 1994;83:701–6.
24 Vottero A, Capelletti M, Giuliodori I et al. Decreased androgen receptor gene methylation in premature pubarche: a novel pathogenetic mechanism? J Clin Endocrinol Metab
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree