7 Oncologic Safety of the Oncoplastic Approach for Breast Conservation Surgery
The increasing popularity of breast oncoplastic surgery has refocused attention on how the partial mastectomy can best be performed to maximize the width of surgical margins at the same time that cosmetic outcome is optimized. The simple “scoop and run” approach to lumpectomy can work well for excision of small malignant lesions. However, unless special surgical procedures are used, saucerization and/or disfiguring deviation of the nipple-areolar complex can result from large breast resections. Ductal carcinoma in situ (DCIS), with or without invasive cancer, commonly follows the segmental ductal anatomy of the breast and can track in narrow long segments (several centimeters in length) from the periphery of the breast toward the nipple.
Most oncoplastic partial mastectomy techniques have not been studied formally in longitudinal studies that address key oncologic issues such as local recurrence rates. Asgeirsson et al 1 reported their intermediate follow-up (up to 4½ years), with local recurrence rates that varied from 0% to 1.8% per year. In 2013, Haloua et al 2 published a literature review of 88 articles on oncoplastic breast-conserving surgery and found that (1) most studies showed significant weaknesses including lack of robust design and important methodological shortcomings, (2) current evidence supporting the efficacy of these oncoplastic procedures is based on poorly designed and underpowered studies, and (3) there is a need for robust comparative studies possibly using randomized controlled trials and/or well-designed multicenter prospective longitudinal studies. The overall oncologic safety of oncoplastic partial mastectomy for cancer therapy is based more on rationale than data, having been extrapolated from the general literature on partial mastectomy. As oncologists, we recognize that such rationale could be logical but inaccurate.
This chapter focuses on the oncologic goals of breast conservation and how oncoplastic procedures for partial mastectomy address these goals. The oncologic problems or issues that may arise with the use of these procedures are also discussed. In addition, the chapter details how careful attention to imaging, surgical margin assessment, and tumor bed marking can optimize the oncologic outcomes. These findings are meant to highlight how surgeons should adapt or modify their decision-making in patient selection for oncoplastic partial mastectomy procedures.
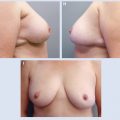
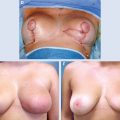
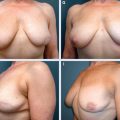
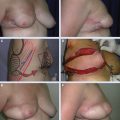
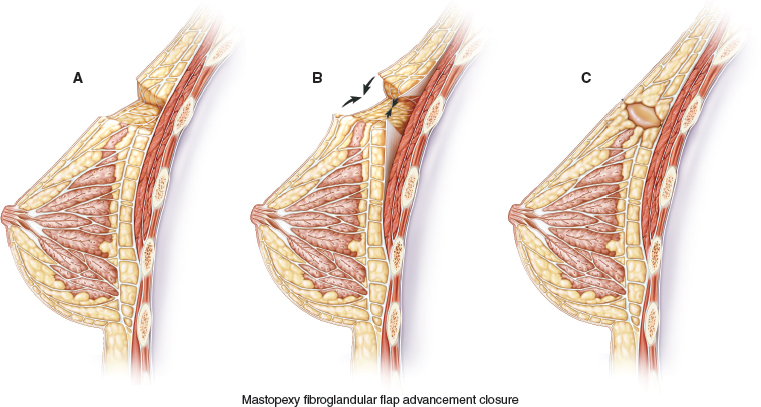
A full-thickness tissue resection is completed (A). The fibroglandular tissue is elevated off the chest wall in preparation for mastopexy closure (B). The flap is advanced and breast tissue is closed at the chest wall, and final skin closure is performed, leaving a small cavity for transient seroma formation that will resolve during radiation therapy (C).
These long segmental cancers can be difficult or impossible to excise with clear margins using traditional lumpectomy techniques. Thus special lumpectomy approaches and closure techniques can be used to follow the segmental contour of the cancer (A). The breast tissue is closed in a way that preserves the breast shape but without moving the nipple-areolar complex on the breast’s skin envelope. The flap advancement and closure of the breast tissue leaves a small cavity for transient seroma formation that resolves during radiotherapy (B and C).
The Oncologic Significance of Oncoplastic Breast Conservation
For breast conservation to be effective, the surgeon needs to obtain complete excision of the cancer with an adequate surgical margin width and achieve a surgical result that maintains the breast’s shape and appearance. 3 The oncoplastic lumpectomy with mammoplasty flap advancement allows the surgeon to perform lumpectomy on larger cancers. 4 The more advanced volume-displacement techniques, which are based on the key principles of breast-reduction surgery, can greatly increase the options for breast conservation in more complex cancer cases. Two oncologic questions arise regarding the application of these procedures: (1) Does the larger size of the primary breast cancer (DCIS or invasive) alter the rate of successful local regional control if the surgical margin width is deemed adequate? (2) Do the volume displacement techniques in any way influence the outcome, as measured by local control of disease at 5 to 10 years?
The NSABP-B-06 trial was designed to assess the treatment outcome for early-stage breast cancer. At entry to this randomized trial, patients had to have a stage I or II cancer measuring less than 4 cm in diameter. 5 Thus, B-06 gives us no direct data about the outcome in treatment of cancers measuring more than 4 cm. Similarly, Veronesi’s breast conservation randomized trial (Veronesi et al 6 ) comparing radical mastectomy to quadrantectomy plus breast irradiation therapy was even more restrictive with cancer size being limited to 2 cm or less.
The landmark randomized trials that are used to justify the use of breast conservation therapy with 20-year follow-up actually do not provide direct evidence that breast conservation with larger (T3) cancers is actually similar to smaller cancers as measured by cancer disease-free and overall survival.
The use of breast conservation therapy with large cancers is really based on extrapolation rather than direct prospective randomized trial results.
Similarly, the oncologic implication of resecting large areas of DCIS has not been explored in prospective randomized trials. The extent of disease for DCIS, as measured by the linear margin width, was originally found to be a predictor of heightened local recurrence rates. In their original reports, the Van Nuys group, led by Silverstein et al, 7 originally suggested that patients with larger DCIS may be best treated with a mastectomy, because their local recurrence rates were high, even when radiotherapy was given. After subsequent reports and data reevaluations, however, it was suggested that the surgical margin width is the more important predictor of local recurrence with DCIS and that the size of the DCIS (larger than 4 cm) is a secondary factor. Restated, if a surgeon can remove the disease en bloc with wide surgical margins, the original dimensions of the disease become relatively unimportant, according to Silverstein. 8
Studies from the 1990s show that BCS was underutilized in the United States, despite clear evidence of the oncologic safety of BCT in multiple settings. 9 , 10 Today, with oncoplastic surgical techniques, the opposite issue needs to be addressed. Larger, partial mastectomy procedures have since been developed and are becoming more and more common. In individual cases, oncoplastic surgeons will use or adapt a variety of oncoplastic approaches—such as batwing mastopexy, radial segmental resection, donut mastopexy, and mastopexy closure—for the partial mastectomy. These oncoplastic approaches are used to creatively address the challenges that may result from variances like breast size, tumor size and location, and degree of breast ptosis—all of which are inevitably seen in breast surgical practices. 4 This variability of oncoplastic technical application, which is part of the surgical artistry of preserving or improving breast shape and appearance, limits the feasibility of developing meaningful randomized trials of different oncoplastic surgical techniques to determine how these compare with standard lumpectomy techniques in terms of breast cancer local recurrence and survival. A careful prospective follow-up of patients will be important in the next 5 to 10 years to confirm that local control is indeed being maintained. In the interim, oncologic safety must be measured by using surrogate markers to predict local recurrence and survival; the most obvious question is whether the width of the surgical margin can be used as an accurate predictor of “complete” surgical excision of the disease.
In 2005, Kaur et al 11 reported a nonrandomized comparative analysis of 30 consecutive patients who underwent oncoplastic partial mastectomies and 30 consecutive patients who underwent standard breast excisions for cancer. They found that the mean volume of the excised specimens was significantly higher in the oncoplastic surgery group (200 cm2 versus 118 cm2) than the standard resection group, demonstrating that more tissue was successfully being removed in the oncoplastic operations. Furthermore, they observed that negative margins (>2 mm) were achieved in 83% of the oncoplastic surgery resections but in only 57% of the standard resections. In a more recent study, Giacalone et al 12 prospectively compared two groups of patients with breast cancers measuring > 15 mm who underwent either a standard partial mastectomy or an oncoplastic resection. The patients who underwent oncoplastic surgery were younger than the standard surgery patients but otherwise, all other demographic and oncological preoperative data were comparable. The investigators observed that (1) the median volume of the excised specimen in the oncoplastic group was higher than in the quadrantectomy group, (2) the nearest lateral margin widths were larger in the oncoplastic group than in the quadrantectomy group, and (3) free surgical margins > 5 mm and > 10 mm were obtained more frequently using oncoplastic surgery than standard partial mastectomy. However, no difference was observed between the two groups in terms of the need for secondary surgeries resulting from problematic surgical margins. These findings confirm, albeit in a nonrandomized fashion, that oncoplastic approaches to the partial mastectomy really do remove more tissue with wider margins while improving the cosmetic outcome.
While the data regarding increased surgical margin width in oncoplastic breast conservation is reasonably persuasive, there is limited published data regarding the use of mammoplasty advancement flap–volume displacement closure (see Fig. 7-1) in relation to subsequent breast cancer local recurrence rates in the breast. Grubnik’s review of therapeutic mammoplasty found reported local recurrence rates of less than 10% with acceptable cosmesis in over 80% of patients. 13 Could the additional incisions made in the breast tissue with mastopexy advancement somehow cause tumor seeding that could later increase local recurrence rates? Other cancers with aggressive local characteristics have been shown to recur locally, such as gallbladder carcinoma recurring in laparoscopic port tracks and phyllodes tumors in the breast. 14
Although tumor seeding is a theoretical concern, it has not been shown to be a significant issue with typical ductal or lobular carcinoma in the breast.
To the contrary, the development of clinically significant needle-track seeding with breast cancer is rarely reported, and in the few studies that describe tumor seeds being histologically detectable in needle sampling tracks, no study has shown these needle tracks to be associated with significantly adverse clinical outcomes. The hypothetical concern of mastopexy advancement seems to be nothing more than theory at present.
Determining the Extent of the Disease
In many circumstances, removing enough tissue to obtain adequate surgical margins may require extensive resections. The lack of surgical planning and forethought can lead to a postoperatively deformed breast. There is value in applying surgical approaches that allow the removal of adequate tissue, yet leave the breast shape and contour intact. To plan an optimal surgical resection, the surgeon should not only understand the distribution of cancer within the breast, but also know the degree to which imaging can accurately predict the true histologic extent and orientation of disease in the breast. In addition to accurate planning, these modalities also minimize the potential for positive margins.
Standard Breast Imaging
The combination of mammography and ultrasonography often helps to predict the orientation and extent of cancer within the breast. Invasive cancers form tumor masses that can be seen well with the two imaging studies used in combination. Ultrasonography complements mammographic imaging of invasive cancers, particularly when masses are concealed by dense fibroglandular tissue on radiographs.
Mammography and ultrasonography can be less reliable in determining the distribution of noninvasive cancers. DCIS can be visualized using mammography when microcalcifications are present and can be seen on radiographs. However, the extent of mammographically visualized calcifications can seriously underestimate the degree of histologic spread when the calcifications form centrally but are absent in the periphery of the cancerous lesion. 15 Because DCIS typically fails to induce masslike changes, ultrasonography is of little or no use in determining the extent of DCIS in preparation for a lumpectomy. One benefit of standard imaging is that it can help to predict the distribution of locally extended cancers; however, it may fail to predict the full extent of segmentally extended cancers in many cases, particularly when they have a significant noninvasive component.
Incomplete excisions are more probable when the mammographic abnormality does not correspond to the entire extent of the lesion. 16 This failed visualization is particularly likely to occur with low-grade DCIS, because microcalcification deposition is absent. 5 With such cases, the surgical margins are often found to be positive at resection, even when specimen radiographic images are obtained and indicate that all disease has been removed. Second operations need to be performed, either with an oncoplastic segmentally orientated reexcision or with mastectomy. Therefore it is important for the surgeon to (1) use all imaging information to carefully assess the location and extent of disease at the time of surgical planning, and (2) consider the possibility of positive margins when performing an initial excision. Ideally, the incision should be placed in a location that will not prevent a cosmetically pleasing reexcision result. A reexcision of cavity margins is usually undertaken through the same incision without any need to extend the incision. A subsequent mastectomy requires an extended or new incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree