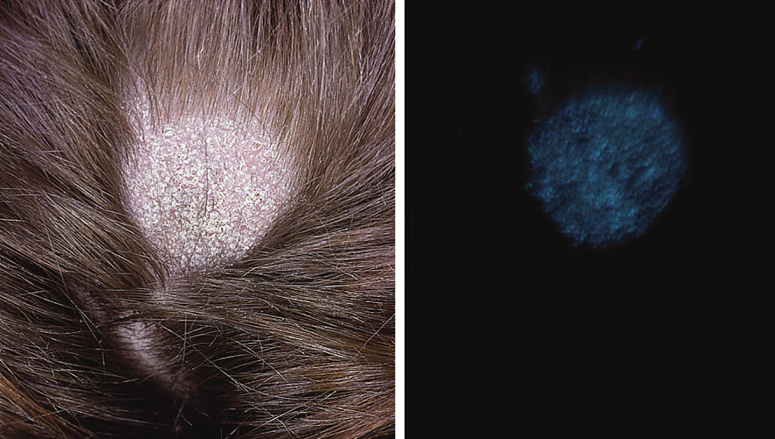
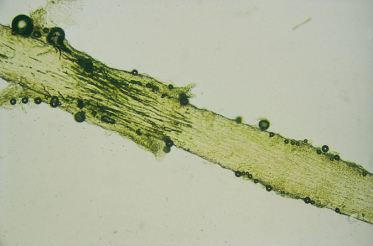
* Infected hairs fluoresce under Woods lamp.
Small-Spored Ectothrix Infections
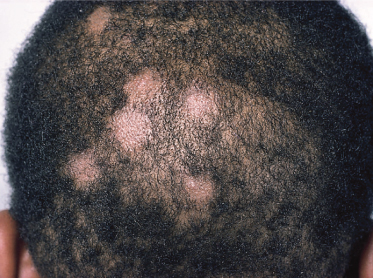
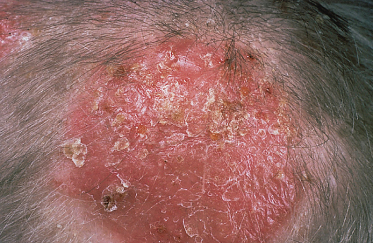
When the causative fungi are anthropophilic (e.g. M. audouinii, M. ferrugineum), slowly extending circular patches with partial hair loss occur in the scalp, with only mild erythema and scaling (Fig. 62.1). More inflammatory lesions with a greater degree of scaling occur when zoophilic or geophilic species are responsible (e.g. M. canis, M. gypseum) (Fig. 62.2).
Large-Spored Ectothrix Infections
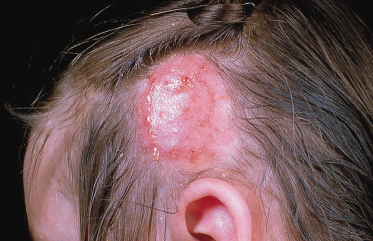
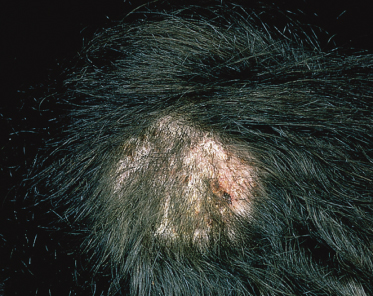
These infections are produced by the zoophilic species complex T. mentagrophytes sensu lato and T. verrucosum and are usually more inflammatory than those caused by M. canis. Solitary lesions are typical with swollen and pustular areas (kerion) containing loose hairs (Fig. 62.3).
Endothrix Infections
Circular patches of alopecia with mild scaling and erythema are characteristic. The infected hairs break off at the surface of the scalp and in dark-haired subjects give a ‘black dot’ appearance (Fig. 62.4). In some patients, scaling becomes widespread with a generalized erythema resembling seborrhoeic dermatitis and leading to progressive hair loss.
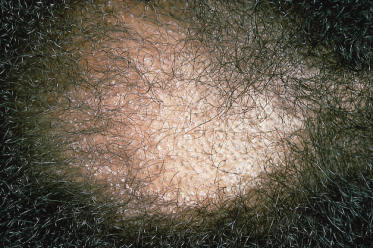
Sometimes a kerion develops followed by scarring and permanent alopecia; this has been seen most frequently when T. violaceum was the invading organism but also occurs in cases of T. tonsurans infection (Fig. 62.5).
Favus, a chronic scalp disease mainly caused by T. schoenleinii, is characterized by crusted, cup-shaped lesions (known as scutula) consisting of hyphae and spores, which develop around follicular openings (Fig. 62.6). Infections frequently produce considerable scarring and alopecia and, unlike other forms of tinea capitis, may persist into adult life. Patients are frequently seen in families, with different generations being infected.
Tinea capitis is generally more common in boys than girls, possibly because shorter hair allows easier access for infecting fungal spores. However, in a survey of fungal infections in the USA, more girls (59%) than boys (41%) were found to have tinea capitis caused by T. tonsurans [13].
The prevalence of a particular dermatophyte as the cause of tinea capitis varies from country to country (Table 62.8). It should be noted, however, that children whose parents and grandparents were immigrants from other countries tend to be infected with the causative fungi from their country of origin, irrespective of how long they have been resident in their adopted country.
Table 62.8 Geographic distribution of principal dermatophytes causing tinea capitis

Tinea Faciei
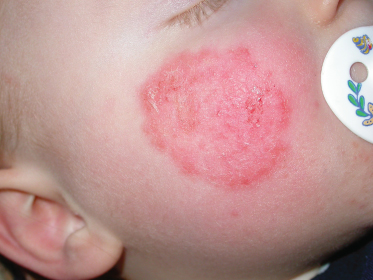
The face is an unusual site for a fungus infection except when representing spread from another site, particularly the scalp. Lesions are similar to those found on other parts of the body (Fig. 62.7). They have been reported in the newborn and infants, and may be caused by anthropophilic or zoophilic species [32–34]. In the USA, it has been reported that the face was the most common site for skin lesions of the body caused by T. tonsurans [13].
Tinea Corporis
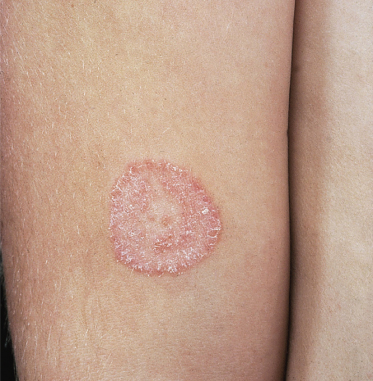
This term is used for infections of the trunk and limbs, which are characterized by circular lesions arising by centrifugal spread of the fungus from the initial site of infection. The degree of inflammation depends on whether the fungus is zoophilic or anthropophilic. A zoophilic species such as M. canis produces erythematous scaly circinate lesions, often with a raised border and pustules; hairs in the involved area may become infected (Fig. 62.8). In contrast, fungi of human origin such as T. rubrum or T. tonsurans cause scaly and not very erythematous lesions, although there is usually a clearly defined margin. Children of all ages can be infected, and rare cases have been recorded in the newborn. Many different fungi have been implicated, including M. canis [35], T. mentagrophytes sensu lato [36] and T. tonsurans [13]. Members of the infant’s family or infected pets are usually the source of infection.
Tinea Incognito
Tinea incognito describes an infection in which the usual clinical signs of tinea have been altered by the application of potent topical steroids. The active margin of the lesion may be lost, pruritus may be decreased or absent, scaling may be absent, and the rash typically becomes widespread. Pustules and papules are prominent [37].
Tinea Imbricata
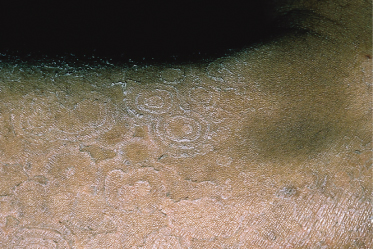
This is a type of chronic tinea corporis caused by T. concentricum. The fungus, and the disease it produces, has a very restricted geographical distribution, being found only in the western Pacific area (e.g. Papua New Guinea), South-East Asia and Amazonia. The infection is often acquired during infancy and occurs in all ages. It is most frequently observed on the trunk and limbs. The lesions are very characteristic, consisting of extensive and persistent rings of concentric scales (Fig. 62.9).
Tinea Cruris
This infection is rare before puberty but is frequently seen in adolescent males. The clinical features are erythematous and scaly lesions extending symmetrically from the groin down the inner surface of the thighs with a marginated edge sometimes containing pustules; lesions are often very itchy. The infection may spread to the perianal area and buttocks. The causative fungus is T. rubrum, T. interdigitale or E. floccosum. The last is almost always the cause of outbreaks in groups of sportspeople or in people living in close communities (e.g. boarding schools) and using shared bathing facilities. Under these circumstances, it is not difficult to isolate the causative fungus from towels, bedding or clothing.
Annular rashes in the napkin area may also be caused by dermatophytes and have to be distinguished from other forms of napkin dermatitis [38,39].
Tinea Pedis (Athlete’s Foot)
This is the most common form of tinea in temperate climates. The causative fungi are all anthropophilic, and T. rubrum is now the most common species isolated in the majority of countries, having in the past three decades supplanted T. interdigitale and E. floccosum.
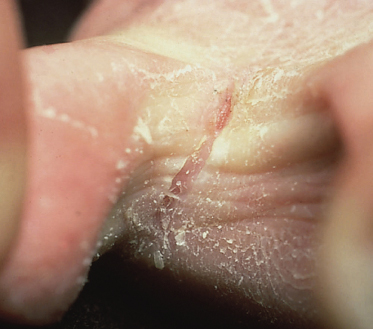
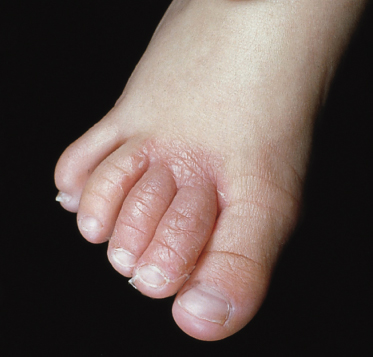
The infection usually starts in the toe spaces, often between the fourth and fifth toe, and is characterized by peeling, maceration and fissures (Fig. 62.10). In the acute phase, the lesions are usually itchy. Spread of the infection may occur to the toes and soles of the feet, where the clinical features depend on the fungus responsible. When T. interdigitale is the causative organism, small clear vesicles may develop which eventually rupture, dry and form an irregular scaly edge. Fine, dry scaling on the soles is typical of T. rubrum infections. These tend to become chronic and widespread, affecting the heels, sides and dorsa of the feet.
The incidence of tinea pedis in children before the age of puberty is low, although there are reports of cases in children less than 4 years old [40,41] (Fig. 62.11). Surveys of schoolchildren in the UK have recorded incidences ranging from 2.2% in 7–10-year-old boys to 6.6% in 11–14-year-old boys [42]. In Denmark, 15-year-old children showed an incidence of tinea pedis of 3.7% [43]. A 20-year survey of tinea pedis in children between 3 and 13 years of age in Italy found only 80 cases compared with over 2500 cases in adults studied concurrently, children therefore representing only 3.1% of cases [44]. A study in France looking at dermatophytosis in children over a 5-year period recorded only two cases of tinea pedis in infants less than 2 years old but 19 cases among children aged between 2 and 10 years [45]. In Korea, a study over a 3-year period examined a total of 102 children with foot dermatitis and reported a total of 21 cases of tinea pedis, based on positive direct microscopy [46]. Seven of the children were less than 4 years old and eight were between the ages of 5 and 9 years. The reason for the different incidences in children and adults is not known. Risk factors such as frequent use of swimming baths are the same in both age groups. Parents of children with tinea pedis are frequently found to have chronic dermatophyte infections. As in adults, tinea pedis occurs more frequently in males than in females. The clinical features in children are similar to those found in adults, although vesicular lesions are perhaps more common and bullous lesions can also occur [47].
Tinea Manuum
This is again rare in children. Lesions usually follow a primary foot infection and T. rubrum is the usual cause. Diffuse hyperkeratosis and peeling of the palms and fingers are the most common features, often affecting only one hand, for reasons that are not understood. Spread may occur to the dorsum of the hand when lesions have a more marked edge.
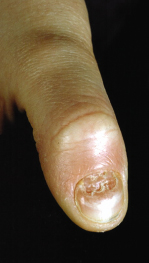
Tinea Unguium
Onychomycosis is uncommon in children, the incidence increasing with age. In a British study, only one case of tinea unguium was found in a survey of 494 schoolchildren aged 5–10 years, an overall prevalence of 0.2% [48]. In the USA, a 3-year survey of children under 12 years revealed 26 cases of onychomycosis [49]. Six children also had tinea pedis. In France, a survey reported four cases of onychomycosis in children up to 2 years old and a further 41 cases in children between 2 and 10 years [45]. Recently in North Poland, onychomycosis in children and adolescents was diagnosed in 99 cases, representing 19.8% of all mycologically confirmed superficial mycoses (500 cases). Fingernail onychomycosis was recognized predominantly in children under 3 years of age (52 cases) while toenail onychomycosis, mostly by T. rubrum, concerned 47 patients. The incidence increased steadily with increasing age [50].
As distinct from adults, most cases of onychomycosis in children show no sign of infection in the adjacent skin sites. However, the parents of children with tinea unguium are frequently found to be infected and therefore are the most likely source of infection [50,51]. Children with infection of a fingernail by an anthropophilic organism causing tinea capitis should be examined for scalp infection.
There are five types of nail involvement.
Distal and Lateral Subungual Onychomycosis.
This is the most common form of fungal nail dystrophy. Invasion starts at the distal end and side of the nail and then spreads proximally up the nail. As the infection becomes established, the nail plate becomes friable and thickened. In developed countries, T. rubrum is the most common cause; in areas where T. tonsurans, T. violaceum or T. schoenleinii is endemic [52], these fungi may produce similar infections.
Superficial White Onychomycosis.
This is an uncommon type of nail damage produced by a fungus invading the superficial surface of the nail plate, with minimal penetration of the nail. White patches that are easily removed by scraping are produced in the nail. This type of infection is mainly seen on toenails and is usually caused by T. interdigitale.
Proximal Subungual Onychomycosis.
Fungal invasion occurs from the skin to the proximal nail bed and white spots occur under the nail; these may extend distally to involve all layers of the nail (Fig. 62.12). It is more often seen with yeast infection and rarely caused by a dermatophyte, but it has been particularly associated with patients with AIDS [53].
Fig. 62.12 Proximal subungual onychomycosis caused by T. interdigitale (zoophilic), assisted by the habit of thumb sucking.
Endonyx Onychomycosis.
This is seen with infection due to dermatophytes that cause endothrix scalp infections, notably the African population of T. rubrum (the former T. soudanense). The nail plate is scarred with pits and lamellar splits. The invasion occurs from the top surface but penetrates deeply into the nail plate [54,55].
Total Dystrophic Onychomycosis.
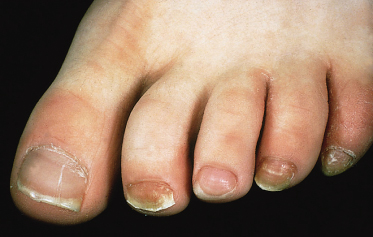
The entire nail plate is destroyed, leaving a thickened and abnormal nail bed. This may occur as a consequence of any of the three types of onychomycosis. Although the majority of toe- and fingernail infections in children are caused by anthropophilic fungi (Fig. 62.13), occasionally zoophilic species such as M. canis [56] and T. equinum [57] are responsible.
Diagnosis.
Diagnosis of a superficial fungal infection is made by microscopic observation of fungal elements in specimens of infected skin, hair or nail. To identify the specific organism, culture may be necessary. It is important to establish the correct diagnosis because clinically indistinguishable lesions may be produced by organisms that are not always responsive to the same form of therapy. For example, T. rubrum and Scytalidium dimidiatum may cause clinically identical infections of the skin and nails but the former is sensitive to griseofulvin, whereas the latter is resistant.
Collection of Specimens
Skin lesions are best sampled using a blunt scalpel. Infected hairs may be removed with flat-ended forceps and infected hair stumps by scraping with a scalpel. Nails are often thickened and nail clippers are necessary to cut off the whole thickness; subungual debris may also be removed with a scalpel. Scalps may be sampled for culture studies by using either a shampoo brush [58] or a toothbrush [59].
Microscopy
Samples are placed in a drop of 30% potassium hydroxide (KOH) on a microscope slide. Softening of the tissue can be hastened by heating gently over a Bunsen burner, but hairs should be allowed to soften without heat so that the arrangement of spores is not destroyed. The incorporation of dimethyl sulphoxide (DMSO) in KOH (DMSO 40 mL, distilled water 60 mL, KOH 30 g) may permit more rapid examination without heating. Unstained preparations are generally satisfactory for the demonstration of fungi in keratin, but chlorazole black E may be added to DMSO to aid in the differentiation of hyphae from common artefacts such as cotton fibres, elastic fibres or ‘mosaic fungus’. The last is an artefact due to deposition of cholesterol and other material around the periphery of the epidermal cells. Calcofluor white (10–40 mg Blankophor®; 95 mL NaOH 0,5M, 5 mL DMSO), a stain that fluoresces green on excitation with UV light, can also be added to an equal volume of KOH. The stain is selective for fungi and is helpful for the detection of scanty fungal elements in clinical material. The preparation must be examined with a fluorescence microscope.
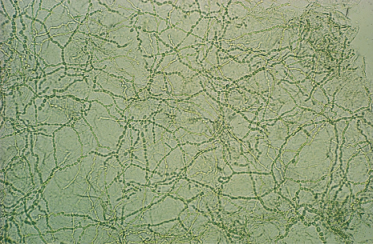
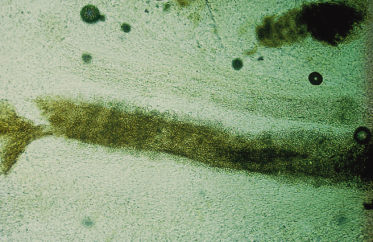
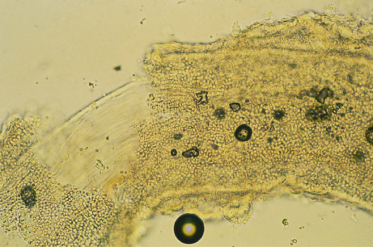
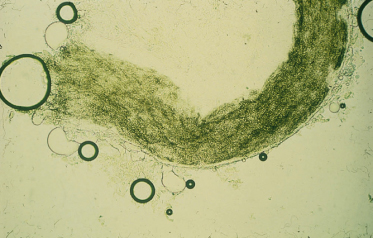
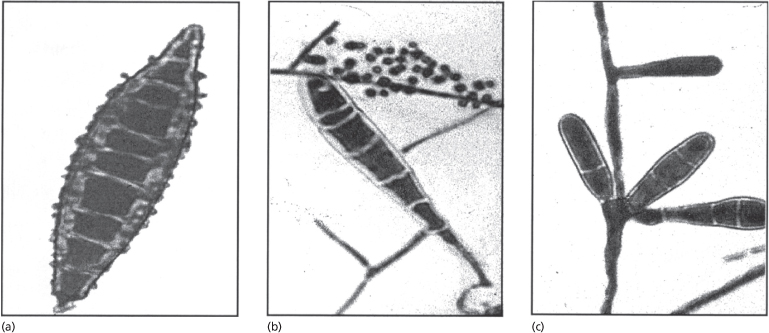
Infected skin scrapings show branching hyphae, often segmenting into chains of arthroconidia (Fig. 62.14). If hairs are infected, the size and arrangement of the spores will help in the identification of the dermatophyte species involved (Figs 62.15–62.18) (see Table 62.7). It should be noted that microscopy of skin, hair and nails is more often positive than culture.
Culture
Petri dishes are very satisfactory for the culture of dermatophytes. Sabouraud dextrose agar (SDA) is the medium most commonly used (peptone 1%, dextrose 4%, agar 1.5%, pH 5.6). Gentamicin or chloramphenicol (0.005%) is added to reduce bacterial contamination and the medium is made selective for dermatophytes by adding cycloheximide (0.05%). Dehydrated media are commercially available, which include these antibiotics either incorporated in the powder (Mycobiotic Agar, Difco/Gibco; Mycosel, BBL) or with the antibiotics in separate vials (Dermasel, Unipath). Dermatophyte test medium (DTM) or so-called Taplin agar are not ideal alternatives because the alkalinization of the media (red colour) is also induced by geophilic dermatophytes (e.g. T. terrestre) or moulds.
The morphology of the dermatophyte colony may indicate the species. The texture of the colony, the surface colour and production of pigments seen on the reverse side of the Petri dish may be diagnostic.
The three genera of dermatophytes are characterized by the type of multicellular spores (macroconidia) produced on culture (Fig. 62.19):
- Microsporum genus: fusiform macroconidia
- Trichophyton genus: cylindrical macroconidia
- Epidermophyton genus: pyriform macroconidia.
The shape and arrangement of unicellular spores (microconidia) and the presence of other structures such as chlamydoconidia, spirals and so on are other features that aid in the identification of a dermatophyte [60].
Molecular Diagnostics
A review of the new species concept of dermatophytes is available [8]. The taxonomic names applied in this chapter are based on this new species concept. Table 62.2 shows the old and new species concepts in this group of fungi. As much as possible, the new conception should be followed, at least when molecular methods for the identification are used. Routine laboratories that rely entirely on conventional diagnostics are unable to differentiate the four species of the T. mentagrophytes complex (T. mentagrophytes sensu lato) that are Trichophyton anamorph of A. benhamiae, zoophilic and antropophilic strains of T. interdigitale, T. erinacei and T. mentagrophytes sensu stricto. Furthermore, it has been shown that misidentifications using phenotypic features are always possible. Given that the former T. mentagrophytes complex consists of four species that are distinguished at many molecular targets, it would be very confusing to use the name T. mentagrophytes for all isolates. The question would arise: which T. mentagrophytes is meant? In turn, species that have been synonymized (e.g. T. raubitschekii, T. megninii and other members of the T. rubrum complex) are not reliably differentiated by any molecular target, inclusive of markers with high discriminating power, such as microsatellites [61]. These factors should be kept in mind by anyone performing dermatophyte identification or reading the results.
As gold standard for the identification of atypical or difficult dermatophyte isolates, as well as from clinical specimens, sequencing of the internal transcribed spacer (ITS) region of the ribosomal DNA is recommended. For detection directly from nail, hair or skin, a nested PCR is sometimes used to increase the sensitivity. The ITS is the only marker for which a complete database exists that can be used for dermatophyte identification (SmartGene, a software package designed by a Swiss company). A ‘barcode’ database at the CBS (www.cbs.knaw.nl) is available free for the public. Attention should be paid, however, to the correctness of species names given in public databases such as NCBI or EMBL because the sequences deposited represent many viewpoints and historical periods. In several cases, only one or two polymorphic nucleotides (signature nucleotides) separate two species, such as M. ferrugineum and M. canis (in ITS2), T. equinum and T. tonsurans (in ITS1 [62]) and the zoophilic and anthropophilic strains of T. interdigitale. Sequences for all dermatophyte species are not available for other gene targets such as LSU (28S of the ribosomal DNA) or the genes for topoisomerase and actin. The chitin synthase (CHS) gene and SSU (18S of the ribosomal DNA) are too conservative for species recognition in dermatophytes.
The drawback of PCR fingerprinting methods (AFLP, AP-PCR, RAPD) in diagnosis is their reliance on culture, which is often unsuccessful in examinations of dermatophytoses. ITS sequencing represents the most valuable means by which species identification can be performed directly from the clinical specimens [63]. Direct sequencing from specimens is applicable, in principle, to other methods and markers as well; however, a complete barcode sequence database is only available for ITS. For all other genomic regions, the extent to which closely related species can be distinguished is not known. At the moment, only sequencing is able to differentiate all species at once, using a panfungal primer pair followed by the seqencing reaction.
All other methods, e.g. PCR-ELISA, real-time PCR or PCR in combination with a blot technique [64–66], depend on the number of species-specific gene probes designed for identification. The advantage of real-time PCR is that post-PCR processing inclusive of the data analysis and quantification of the copy number of the target is performed automatically, saving laboratory resources and time. In addition, the method has a low risk of contaminations. It is, however, an expensive method if the diagnosis involves a broad spectrum of possible species. The other two methods are less expensive because a single panfungal PCR is enough for identification. The disadvantage is that post-PCR processing (ELISA, blot) is more laborious and time consuming than real-time PCR. The higher time requirement of a few hours is not really a drawback because dermatophytoses are not life-threatening.
However, none of these methods is able to discriminate between closely related species, e.g. T. tonsurans and T. equinum, T. mentagrophytes sensu stricto and T. schoenleinii, M. canis and M. ferrugineum as well as the African population of T. rubrum and the remaining strains of this species. Furthermore, the zoophilic and anthropophilic strains of T. interdigitale are not distinguished which is important for therapy and co-treatment of the animal source. The only kit commercially available (ONYCHODIAG kit) is manufactured by Bio-Advance and employs just a single probe to detect all dermatophytes as a group, inclusive of non-pathogenic geophiles and related chrysosporia [67]. This makes the distinction of zoophiles from anthropophiles impossible, a factor that is important in therapy. Another advantage of ITS sequencing is that most other fungal species (non-dermatophytes) involved in dermatomycoses can also be identified [68].
Differential Diagnosis
Tinea Capitis
Scalp scaling without hair loss occurs in a number of conditions. Seborrhoeic dermatitis, psoriasis or pityriasis amiantacea may appear similar to tinea capitis. In pityriasis amiantacea, large numbers of thick scales are present around hairs, which do not break. In alopecia areata, where there are patches of hair loss, there is usually no scaling and a number of broken tapering hairs (‘exclamation mark’ hairs) are present. Dermatoscopy may be helpful in differentiating tinea capitis (‘comma hairs’) from alopecia areata (exclamation mark hairs) [69].
Tinea Corporis
Tinea corporis may be mistaken for other annular lesions such as eczema, annular erythemas, psoriasis and granuloma annulare. Dermatophyte infections usually have a distinct edge where scaling is more obvious and the lesions itch.
Tinea Cruris
Tinea cruris should be differentiated from flexural psoriasis, seborrhoeic dermatitis, erythrasma and Candida intertrigo.
Tinea Pedis
Other causes of interdigital scaling are Candida infection, erythrasma, Gram-negative and staphylococcal infections.
Tinea Manuum
Tinea manuum should be distinguished from eczema and psoriasis.
Tinea Unguium
Psoriasis, eczema and Candida infection of the nails should be considered in the differential diagnosis of tinea unguium. Features of psoriasis of the nail are involvement of the same nails of the opposite hand or foot, pitting of the nails and onycholysis; psoriasis is often present elsewhere.
Treatment.
Although certain forms of dermatophytosis are self-limiting, most types require treatment. Topical therapy is normally used for localized lesions not involving hair or nails. Oral therapy is essential for chronic and widespread infections and for those involving the scalp or nail.
The main antifungal drugs used in the treatment of dermatophyte infections include the following.
Griseofulvin
This is an antifungal antibiotic derived from a number of Penicillium species. It is fungistatic and affects nuclear division by binding to microtubular proteins. It also acts as an inhibitor of nucleic acid synthesis. It is selectively concentrated in the stratum corneum, where it inhibits fungal growth sufficiently to prevent invasion of newly formed keratin. Griseofulvin was the first oral drug available for the treatment of dermatophytosis. Its activity is restricted to the dermatophytes and its clinical use is limited to infections caused by these fungi. Given the superior activity obtained with the newer agents, griseofulvin is rarely used now except for some scalp infections.
Azoles
These are synthetic drugs that act by inhibition of ergosterol biosynthesis by fungal cytochrome P450 enzymes. Ergosterol is an essential constituent of the fungal cell membrane. The imidazoles contain a large number of compounds primarily for topical use such as clotrimazole, miconazole, econazole, bifonazole, isoconazole, oxiconazole, sulconazole, terconazole, tioconazole, fenticonazole and ketoconazole. They have a broad spectrum of antifungal activity including dermatophytes and yeasts such as Candida and Malassezia species. Some also show activity against Gram-positive bacteria.
The triazoles itraconazole and fluconazole are used orally for the treatment of superficial fungal infections, having a similar broad spectrum of activity to the imidazoles. In many countries they are not licensed at present for use in children.
Allylamines
These synthetic compounds block the formation of ergosterol in the cell membrane by inhibition of squalene epoxidase. They have a broad spectrum of activity in vitro. Naftifine shows antifungal activity against both dermatophytes and yeasts and is used topically in the treatment of superficial fungal infections. It also has anti-inflammatory activity. Terbinafine is available for topical and oral use. Although topically effective against both yeasts and dermatophytes, terbinafine, when given orally, is effective only against dermatophytes. Against these fungi, it shows primary fungicidal activity. Oral terbinafine is licensed for use in children only in some countries. It is available as a cream, solution, a 250 mg tablet, and in the form of packaged granules. A 125 mg tablet is now available in some countries for treating children.
Morpholines
These compounds strongly inhibit ergosterol biosynthesis at two different enzymatic stages specific for fungal cells (the reductase and isomerase stage). Amorolfine, a phenyl propyl morpholine derivative, has in vitro activity against a variety of fungi including dermatophytes and yeasts; it has fungicidal activity against dermatophytes. It is inactive when given systemically and is only used topically.
Miscellaneous
Other miscellaneous compounds available for topical treatment include tolnaftate, ciclopiroxolamine, haloprogin and derivatives of undecylenic acid.
Treatment of Specific Conditions
Tinea Capitis.
Oral therapy is essential. Adjuvant therapies such as antifungal shampoos or topical antimycotics should be routinely combined. At present, griseofulvin is the only antifungal licensed for oral use in tinea capitis in most parts of the world. Griseofulvin (10 mg/kg daily) should be given for at least 6 weeks. In some cases, particularly with T. tonsurans infections, longer courses and sometimes higher doses (20 mg/kg daily) of griseofulvin therapy may be needed [22]. M. canis infections may also need longer courses [70]. In a meta-analysis of clinical trials of griseofulvin use in tinea capitis, the overall mean effective cure at 4–6 weeks post treatment was 73.4% ± 7%. Higher efficacy rates appeared to be reported with the use of higher dosages of griseofulvin (>18 mg/kg/d). When broken down by species, the mean efficacy rates for Trichophyton and Microsporum were 67.6% ± 9% and 88.1% ± 5% respectively [71]. The drug should be taken after meals. An oral suspension is available in some countries.
Adverse reactions that have been reported with griseofulvin include drowsiness, headache and nausea; photosensitivity and urticarial reactions are also occasionally observed. The use of selenium sulphide shampoo, 2.5% twice weekly, has been shown to reduce the frequency of positive cultures from scalps when used with griseofulvin [72]. Ketoconazole shampoo may also be helpful in conjunction with griseofulvin.
The use of the allylamine terbinafine and the triazoles itraconazole and fluconazole in the treatment of tinea capitis has been reviewed by Gupta et al. [73], who summarized many of the open study and trial data. These drugs generally appear to have equal efficacy with griseofulvin, and are effective with shorter courses of therapy.
Few significant adverse effects have been reported. As active levels of drug remain in the keratinized tissues for weeks after therapy has ceased, pulse and intermittent therapy have been reported to be successful with itraconazole and fluconazole.
Terbinafine has been shown to be effective in the treatment of tinea capitis with Trichophyton species. The following dosages are commonly recommended:
- children weighing <20 kg, 62.5 mg
- children weighing 20–40 kg, 125 mg
- children weighing >40 kg, 250 mg.
With T. tonsurans, doses are generally given once daily for 4 weeks. Some reports suggest that a 2-week course may be effective. The use of terbinafine in infants and neonates has been limited [74,75]. Terbinafine is well tolerated in children between 2 and 17 years old, with few adverse events. Gastrointestinal discomfort and skin rashes have been reported. There is some evidence that terbinafine is less effective in infections caused by Microsporum species and that these may need longer courses of therapy and higher doses [76–80]. In a comparative study of a new paediatric formulation of terbinafine hydrochloride oral granules with griseofulvin oral suspension, clinical cure was higher for terbinafine than for griseofulvin, but the difference was not found to be statistically significant. Subgroup analyses revealed that terbinafine was significantly better than griseofulvin among patients with T. tonsurans and vice versa with M. canis [81].
Itraconazole is also not yet licensed for oral use in tinea capitis in many countries but has been used quite extensively in open studies and trials. It is available in capsule form or as a suspension. It should not be given with a meal and can cause diarrhea because of the presence of cyclodextrin. For continuous therapy, itraconazole is generally given as 3–5 mg/kg per day for 4–6 weeks. In the majority of the studies, the efficacy was high. In one study, 50 children from six countries with tinea capitis caused by either M. canis or T. tonsurans were included. The majority of cases were treated with 100 mg of itraconazole daily for approximately 5 weeks [82]. In total, 38 patients (76%) were considered healed at the end of the study. Another study [83] compared 100 mg daily itraconazole and 500 mg daily griseofulvin in the treatment of tinea capitis. There were 17 patients in each group, the majority of infections being caused by M. canis. Treatment was given for 6 weeks, and at follow-up 8 weeks after the end of therapy, 80% of patients in each group were cured. However, another study in which 25 patients with T. tonsurans infection completed a course of 4 weeks of 100 mg itraconazole daily together with selenium sulphide shampoo resulted in only 10 cures (40%) [84]. In one study, 163 children with tinea capitis cause by M. canis and previous unsuccessful therapy with terbinafine were treated with 5 mg/kg/day given in a continuous regimen either as a capsule or an oral suspension. In all children, there was both clinical and mycological cure after a mean treatment period of 39 ± 12 days [85]. In children in their first year of life, itraconazole proved to be a safe and effective treatment option for M. canis-induced tinea capitis [86].
Pulse therapy has been reported to be successful. In a small study involving 10 children [87], the subjects took 5 mg/kg itraconazole daily for 1 week followed by 2 weeks off between consecutive pulses. One, two or three pulses resulted in clinical cure in one, six and three patients respectively. A larger study followed which produced a mycological and clinical cure in 30 out of 37 patients (81%) [88].
Few data are available for the use of fluconazole in tinea capitis. In one study, 48 patients with Trichophyton tinea capitis were treated with 6 mg/kg daily for 2 weeks, followed by 2 weeks without treatment. At 4 weeks after the start of therapy, they were examined and those still with clinical signs were given a further week’s treatment. Follow-up at 12 weeks resulted in clinical and mycological cure in 37 out of 42 patients (88%) [89]. A subsequent larger study, including patients with both Trichophyton and Microsporum infections, contained 61 children, dosed at 8 mg/kg once weekly. At follow-up, complete clinical and mycological cure was seen in 60 patients; of these, 47 were treated for 8 weeks, 10 for 12 weeks and three for 16 weeks. In total, three patients suffered a mild, reversible gastrointestinal complaint and one patient showed an elevated liver function test, but this was asymptomatic and reversible [90]. In a recent study, fluconazole at two different doses (6 mg/kg per day for 3 weeks followed by 3 weeks of placebo, fluconazole 6 mg/kg per day for 6 weeks) was compared with standard-dose griseofulvin (11 mg/kg per day microsize formulation) in tinea capitis caused mainly by T. tonsurans (86%, 11% M. canis). At the end of treatment, both regimens produced low mycological and clinical cure rates. Mycological cure rates for fluconazole at 3 weeks and 6 weeks were 44.5% and 49.6% respectively. For griseofulvin the mycological cure rate was 52.2% [91].
A study compared griseofulvin with terbinafine, itraconazole and fluconazole in the treatment of Trichophyton tinea capitis [92]. In total, 200 patients were randomized to the four groups. Griseofulvin therapy was 20 mg/kg per day for 6 weeks; the remaining antifungals were given for 2 weeks and the patients were assessed at 4 weeks and, if clinically indicated, a further 1 week of treatment was given. Terbinafine was given as follows: >40 kg, 250 mg per day; 20–40 kg, 125 mg per day; and <20 kg, 62.5 mg per day. The itraconazole dose was 5 mg/kg per day and fluconazole 6 mg/kg per day. At 12 weeks’ follow-up, effective treatment was seen in 92% of the griseofulvin group, 94% of the terbinafine group, 86% of the itraconazole group and 84% of the fluconazole group. Overall, 6 weeks of griseofulvin therapy appears similar in efficacy to 2–3 weeks’ therapy with the newer antifungals.
Tinea Corporis.
Unless there is widespread infection, in which case oral griseofulvin should be given for at least 4 weeks, lesions usually respond well to a topical imidazole, such as clotrimazole, or to ciclopiroxolamine, amorolfine or terbinafine. These compounds are available as creams and lotions and most will be effective after once- or twice-daily application for 2–4 weeks. Terbinafine cream is effective after 7 days of treatment applied once daily [93].
Tinea Cruris.
Topical therapy is as indicated above for tinea corporis.
Tinea Pedis.
Tinea pedis will usually respond to topical treatment with one of the compounds mentioned above. The antifungal should be applied twice daily to the toe clefts for at least 2 weeks; longer treatment is sometimes required. However, a comparison of 1 week of terbinafine 1% cream with 4 weeks of clotrimazole 1% cream found the former antifungal to be more effective [94]. Recent studies have shown that even a single dose of terbinafine 1% film-forming formulation is as effective as the 1-week course of terbinafine 1% cream [95].
If a mixed fungal and bacterial infection is suspected, imidazoles such as clotrimazole, miconazole or econazole, which possess some antibacterial activity, are preferable. If tinea pedis is of the dry scaly type, 4 weeks of topical treatment would be the minimum time to produce remission and the relapse rate is often high. Oral griseofulvin is the only licensed alternative for children in many countries, but both oral itraconazole and terbinafine have been shown to be effective in this type of infection [96]. Oral terbinafine granules have recently been approved for use in children 4 years of age or older for the treatment of tinea capitis at the following doses: <25 kg, 125 mg once daily; 25–35 kg, 187.5 mg once daily; >35 kg, 250 mg once daily.
Tinea Unguium.
This rare condition in children responds well to oral terbinafine even although there have been few clinical trials and in many countries, the drug is not licensed for use in children. Similarly, studies of itraconazole and fluconazole for the treatment of nail disease in children are limited. Although there is faster nail growth in children and the success rates with oral therapy should be higher, responses are often disappointing. The use of these newer antifungals in the treatment of onychomycosis in children has been reviewed by Gupta et al. [97].When oral therapy is contraindicated, application of an antifungal nail lacquer could be considered. Ciclopirox nail lacquer applied once daily for up to 6 months up produces varying mycologic cure rates from about 30% up to 85,6% [98].
Alternatively, amorolfine 5% nail lacquer applied once or twice weekly for up to 6 months produces cure in approximately 40–55% of patients with mild onychomycosis without nail matrix involvement. With both of these compounds, better results are obtained if they are used in conjunction with oral griseofulvin [99]. Studies in adults suggest that even better responses can be obtained by combining amorolfine with either terbinafine or itraconazole [100].
References
1 Remak R. Zur Kenntnis der pflanzlichen Natur der Porrigo lupinosa. Med Zeitung 1840;9:73–4.
2 Schoenlein JL. Zur Pathologie der Impetigines. Arch Anat Phys Wiss Medical 1839:82.
3 Remak R. Diagnostische und Pathogenetische Unterschungen in der Klinik des Geh. Raths Dr Schoenlein auf dessen Veranlassung angestellt und mit Benutzung anderweitiger Beobachtungen veröffentlicht. Berlin: A. Hirschwald, 1845: 242.
4 Gruby D. Sur les mycodermes que constituent la teigne faveuse. CR Acad Sci (Paris) 1841;13:309–12.
5 Sabouraud R. Les Teignes. Paris: Masson, 1910.
6 Emmons CW. Dermatophytes: natural groupings based on the form of spores and accessory organs. Arch Dermatol Syph 1934;30:337–62.
7 Stockdale PM, MacKenzie DWR, Austwick PKC. Arthroderma simii sp. nov.: the perfect state of Trichophyton simii (pinoy) comb. nov. Sabouraudia 1965;4:112–23.
8 Gräser Y, Scott J, Summerbell R. The new species concept in dermatophytes – a polyphasic approach. Mycopathologia 2008;166:239–56.
9 Gentles JC. Experimental ringworm in guinea pigs: oral treatment with griseofulvin. Nature (Lond) 1958;182:476–7.
10 Prochacki H. Mycological flora isolated from people in Poland. Mycopathol Mycol Appl 1970;40:65–72.
11 Sinski JT, Flouras K. A survey of dermatophytes isolated from human patients in the United States 1979–1981 with chronological listings of worldwide incidence of five dermatophytes often isolated in the United States. Mycopathologia 1984;85:97–120.
12 Verhagen AR. Distribution of dermatophytes causing tinea capitis in Africa. Trop Geogr Med 1974;26:101–20.
13 Bronson DM, Desai DR, Barskey S et al. An epidemic of infection with Trichophyton tonsurans revealed in a 20-year survey of fungal infections in Chicago. J Am Acad Dermatol 1983;8:322–30.
14 Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004;50:748–52.
15 Rogers M, Muir D, Pritchard R. Increasing importance of Trichophyton tonsurans in childhood tinea in New South Wales. Australas J Dermatol 1993;34:5–8.
16 Hay RJ, Robles W, Midgley G et al., on behalf of the European Confederation of Medical Mycology Working Party on Tinea Capitis. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol 2001;15:229–33.
17 Borman AM, Campbell CK, Fraser M et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol 2007;45:131–41.
18 Ginter-Hanselmayer G, Weger W, Ilkit M et al. Epidemiology of tinea capitis in Europe: current state and changing patterns. Mycoses 2007;50 (Suppl 2):6–13.
19 MacKenzie DWR. The extra human occurrence of Trichophyton tonsurans in a residential school. Sabouraudia 1961;1:58–64.
20 Clayton YM. Scalp ringworm. In: Verbov JL (ed) Superficial Fungal Infections. Boston: MTP Press, 1986: 1–19.
21 Babel DE, Baughman SA. Evaluation of adult carrier state in juvenile tinea capitis caused by Trichophyton tonsurans. J Am Acad Dermatol 1989;21:1209–12.
22 Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol 2000;42:1–20.
23 White JM, Higgins EM, Fuller LC. Screening for asymptomatic carriage of Trichophyton tonsurans in household contacts of patients with tinea capitis: results of 209 patients from South London. J Eur Acad Dermatol Venereol 2007;21:1061–4.
24 Munro-Ashman D, Clayton YM. Tinea pedis in adolescence. Proc Roy Soc Med 1962;55:551–4.
25 English MP. The developmental morphology of the perforating organs and eroding mycelium of dermatophytes. Sabouraudia 1967–8; 6: 218–27.
26 Allen AM, King RD. Occlusion, carbon dioxide and fungal skin infections. Lancet 1978;1:360–2.
27 Kligman AM. The pathogenesis of tinea capitis due to Microsporum audouinii and M. canis. J Invest Dermatol 1952;18:231–46.
28 Rasmussen JE, Ahmed AR. Trichophyton reactions in children with tinea capitis. Arch Dermatol 1978;114:371–2.
29 Almeida SR Immunology of dermatophytosis. Mycopathologia 2008;166:277–83.
30 Vermout S, Tabart J, Baldo A, Mathy A, Losson B, Mignon B. Pathogenesis of dermatophytosis. Mycopathologia 2008;166:267–75.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree