Deer are strongly associated with the presence of ticks. They are preferred feeding hosts for adult female ticks, who mate after feeding, drop back into the undergrowth and lay between 1000 and 2000 eggs before dying. Deer are important in the maintenance and expansion of tick populations because of their feeding role at the tick’s reproductive stage but they are not competent reservoir hosts for B. burgdorferi. Increased numbers and geographic ranges of deer in some regions of Europe and North America have been linked to significant increases in tick populations and Lyme borreliosis incidence.
Human beings can be incidental feeding hosts for ticks. Larval ticks pose minimal risk for borrelial transmission, as it is rare for them to have been infected transovarially. Nymphal ticks are most likely to transmit infection to people, as they are very small (about the size of a poppyseed) and may not be noticed, even after feeding. Their major feeding period is during late spring and early summer, when human outdoor recreational activity is also likely to be at its peak. The larger adult ticks are more likely to be spotted and removed before completing their feeds (Fig. 59.2).
Most ticks do not carry borreliae. Infected I. ricinus ticks are unlikely to transmit infection in the first 18–24 hours of feeding and I. scapularis ticks within the first 36 hours, but transmission risk rises steadily as feeding continues. Early removal of attached ticks greatly reduces human infection risk [4,6]. There is some evidence that transmission may take place at an earlier stage during I. persulcatus blood meals [6].
Most cases of Lyme borreliosis are reported in the summer months, between May and September, but some patients with disseminated and later presentations are diagnosed at other times of the year. In many studies there is a bimodal age distribution, with higher incidences in the 5–19 year age group and in people over age 40. Male:female incidence ratios seem to be about equal or with a slight preponderance of cases in males in some studies [3,4,6–8]. The major risk factors for infection are residential or recreational activities in tick habitats.
Causative Organisms and Pathogenetic Factors.
At least 15 genospecies of Borrelia burgdorferi sensu lato have been identified; the majority are not pathogenic. The only pathogenic genospecies found in North America is B. burgdorferi sensu stricto. A wider range of pathogenic genospecies is found in Europe, predominantly B. garinii and B. afzelii, but B. burgdorferi sensu stricto occurs in some areas [10,11]. All pathogenic strains can cause erythema migrans, the early skin lesion. There is evidence for some genospecies-dependent variations associated with later manifestations and for within-genospecies variation in pathogenicity [12]. B. burgdorferi sensu stricto is strongly linked with arthritic and neurological complications, B. garinii and B. bavariensis with neuroborreliosis and B. afzelii with acrodermatitis chronica atrophicans (ACA). Occasional cases of erythema migrans are caused by B. spielmanii and there have been only rare reports of disease linked to B. valaisiana and B. lusitaniae. Genospecies differences also have implications for vaccine development.
Variations in the geographic distribution of European borrelial genospecies affect the incidence and types of clinical presentations of later disease seen in different locations. For example, ACA frequently occurs in Scandinavia and central Europe, where B. afzelii is commonly found. The condition is infrequently seen in the UK, where B. afzelii is uncommon. The most commonly identified pathogenic genospecies in the UK is B. garinii, and acute neuroborreliosis is the main complication seen there. A high proportion of infected ticks in the UK carry B. valaisiana, which is essentially non-pathogenic. This may have some bearing on the lower overall incidence of clinically significant disease there compared to other parts of Europe where a higher proportion of infected ticks carry more pathogenic genospecies.
Borrelia burgdorferi is an obligate parasite with limited biosynthetic ability, relying on its host for many nutrients. It does not possess the virulence factors such as toxins, lipopolysaccharides and enzymes that are associated with many bacterial pathogens [10]. Borreliae are highly motile and can disseminate through tissues and bind closely to host cell surfaces, affecting their function [13].
Borreliae must adapt to life in very different environments: the midgut of the tick vector at ambient temperature and the mammalian or avian natural reservoir host from about 35o to 39oC. They vary in gene expression, leading to production of different protein components. Some help the organisms to evade the initial defences of the host’s innate immune system, others contribute to evasion of the subsequent acquired immune response, at least for some time. For example, borreliae express an outer surface protein (Osp) called OspA within the tick, but OspC is preferentially expressed during the early stages of mammalian. Expression of another protein, VlsE, which has an elaborate system for extensive variation over time, is required to maintain persistent infection. Synthesis of VlsE starts at about the time that OspC production ceases and helps the organism to evade the host’s humoral immune response, at least for some time [10,13].
Many clinical manifestations of Lyme borreliosis occur largely as a consequence of the human immunopathological response to infection. Some borrelial lipoproteins can activate a variety of cells, including macrophages, B-cells, dendritic cells and endothelial cells, triggering inflammatory responses that contribute to pathogenesis [10,13,14]. There is also some in vitro evidence that antibodies produced in response to B. burgdorferi infection may also bind to human neural and other antigens [13].
Ixodid Tick Bites
Ixodid ticks attach to their hosts by barbed mouth parts. Their saliva contains anaesthetic, anti-inflammatory and anticoagulant substances that allow them to attach and complete their feed without their hosts becoming aware of their presence [15]. The bites are not usually significantly painful or itchy, although some people who have had many bites over a prolonged period, such as forestry workers, can become sensitized to tick saliva and develop itchy skin reactions. This can provide some protection against B. burgdorferi infection, by drawing early attention to the tick before there is significant risk of spirochaetal transmission.
Clinical Features.
Borrelia burgdorferi infection can be asymptomatic or minimally symptomatic, as shown by prospective studies in areas of high prevalence [7,8,16,17]. Clinically significant disease has been customarily divided into three stages – early localized, early disseminated and late Lyme borreliosis – but the process should be regarded as a continuing pathological evolution in some untreated patients rather than having distinct phases or processes. Progression to later stage disease is not inevitable. The most frequently affected tissues and organs are the skin, nervous system and joints. Case definitions have been published, primarily for epidemiological purposes in the USA and for clinical use in Europe [18,19].
Skin Manifestations
Erythema Migrans (Figs 59.3, 59.4).
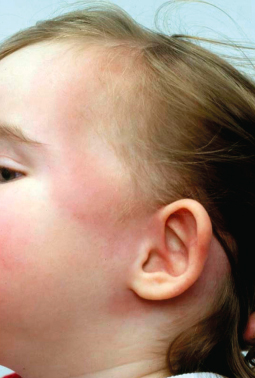
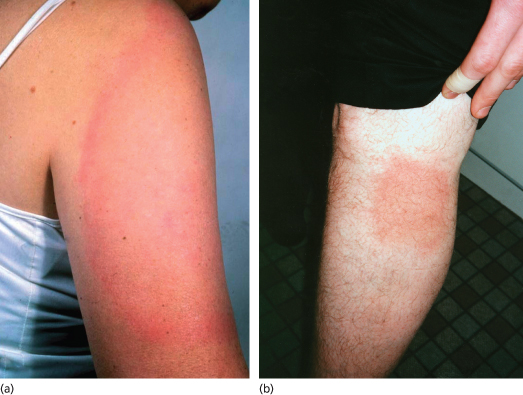
Erythema migrans (EM) is the most characteristic presentation of Lyme borreliosis, occurring in about 90% of symptomatic infections [7,8,20]. It is an erythematous, usually annular lesion spreading slowly from the site of a tick bite (often unnoticed) that had occurred 2 or more days previously. This lag phase occurs because spirochaetal replication is relatively slow by comparison to pyogenic bacteria. Localized redness appearing within a few hours of a tick bite is likely to be caused by an immediate inflammatory response or hypersensitivity reaction and usually fades within several days. Common sites for tick attachment and undisturbed blood meals which can result in borrelial transmission include skinfolds (groins, armpits, backs of knees, waistband area, under breasts), back and scalp. Tick bites are more frequent around the head and neck areas of children than adults.
Most EM rashes become evident within about 5–14 days of a bite. They are usually round or oval but some can have a more triangular or linear appearance, presumably determined in part by lines of skin tension. A central punctum may be present at the tick attachment site. The leading edge can have a stronger colour, giving the appearance of an outer ring or border, but is not significantly raised. They are not usually particularly pruritic or painful [20]. Very itchy or painful lesions should raise suspicion of other conditions, including severe insect bite reactions or pyogenic infections. Most EM rashes are homogeneous in the early stages but with longer duration, some can develop an area of central clearing, giving a so-called ‘bull’s-eye’ or target-like appearance. Very pale lesions may be seen more easily when the skin is warm, e.g. after exercise or bathing. If left untreated, EM rashes eventually resolve over weeks to months, but usually clear within a few days of commencing appropriate treatment.
There can be variations in EM appearance, related to the infecting genospecies. In North American-acquired infections, caused exclusively by B. burgdorferi sensu stricto, rashes tend to evolve more quickly and are less likely to have central clearing than European-acquired infections caused by B. afzelii and are more likely to be accompanied by systemic symptoms and signs including malaise, myalgia, arthralgia, headache, fever and regional lymphadenopathy [21]. Some have central vesiculation, which can cause difficulties in differentiation from cellulitis, arthropod bites or herpesvirus infections [22]. Some EM lesions caused by B. afzelii develop slowly, with little systemic upset, and can reach large sizes with high rates of central clearing. Infections caused by B. garinii seem to be more virulent than those caused by B. afzelii, with more homogeneous and faster evolving rashes and a higher likelihood of systemic symptoms and signs [23].
Multiple erythema migrans can occur, principally in infections caused by B. burgdorferi sensu stricto. Affected patients have multiple smaller lesions resulting from haematogenous spread of spirochaetes from the initial lesion. They usually have significant systemic symptoms and may also have other objective extracutaneous manifestations of Lyme borreliosis, which include facial nerve palsy, meningitis, carditis and arthritis [24].
Differential diagnosis of erythema migrans includes reactions to tick bites or other arthropod bites, urticaria, cellulitis, granuloma annulare, ringworm, herpes simplex or zoster, fixed drug eruptions or contact dermatitis [20,25,26]. The histological appearance of erythema migrans is characterized by patchy perivascular infiltrates, predominantly lymphocytic, with plasma cells, mast cells and occasionally eosinophils, mainly affecting the superficial dermis. Immunohistochemical staining may demonstrate occasional spirochaetes, but silver staining is prone to artefact [27,28].
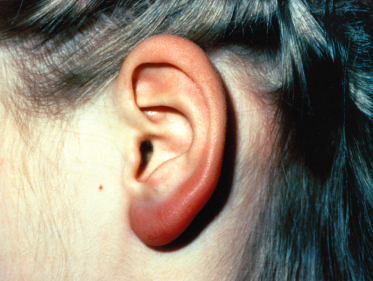
Borrelial Lymphocytoma (Fig. 59.5).
Borrelial lymphocytoma (also termed lymphadenosis benigna cutis) is an uncommon early manifestation of European Lyme borreliosis; only rare cases have been reported in the USA. It may develop several weeks to months after a tick bite and occurs more frequently in children than adults. It presents as a bluish-red tumour-like skin infiltrate several centimetres in diameter. The most common sites are earlobe, ear helix, nipple or scrotum. If untreated, it can persist for some months but usually resolves rapidly with antibiotic treatment [29,30]. Histological examination shows dense lymphocytic and histiocytic infiltrates, frequently with germinal centres [31]. Borrelial lymphocytoma lesions have occasionally been misdiagnosed as B-cell lymphomas.
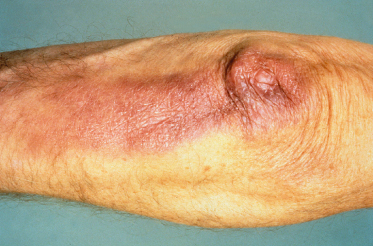
Acrodermatitis Chronica Atrophicans (Fig. 59.6).
Acrodermatitis chronica atrophicans (ACA) is an uncommon manifestation of long-standing infection, seen almost exclusively in European-acquired infections. It occurs mainly in older people, with only rare reports of ACA-like lesions in children [2,32]. Most cases are caused by B. afzelii infection. It usually occurs on extensor sites of the extremities, most commonly on the lower legs, causing localized livid red colour changes, doughy swelling and induration of the affected skin. Untreated lesions may progress over years, with development of hyperpigmentation and atrophy, giving a cigarette paper-like appearance [2]. There may be an associated peripheral neuropathy. If severe, this can lead to joint damage secondary to the neurological deficit. The differential diagnosis depends on the duration of the condition and includes vascular insufficiency, acrocyanosis, livedo reticularis, lymphoedema or chilblains. The infection responds to treatment but completeness of recovery depends on the degree of underlying tissue damage. Histological appearance depends on the duration of the lesion, with earlier inflammatory lesions showing perivascular infiltrates of lymphocytes and plasma cells, dermal oedema and telangiectasia. Epidermal atrophy and loss of epidermal appendages are characteristic findings in late-stage ACA [28,33].
Other Skin Manifestations.
Several other skin conditions, including morphea and lichen sclerosis et atrophicus, have been linked to Borrelia burgdorferi infection in a few case reports and small studies, predominantly from high-endemic areas of Europe. Other European and North American studies have shown no link with these conditions. These mainly early reports should be interpreted cautiously, as the laboratory tests used in support of some cases relied on methods such as silver staining, which is prone to artefact, or IgM antibody tests, which are known to have a significant risk of false-positive reactions. Positive serology, especially in areas of high prevalence (where background seroprevalence in the population can be as high as 10%), should not be interpreted as evidence for causation of atypical lesions without additional support from direct detection of the organism. More recent studies and reviews suggest that B. burgdorferi is rarely associated with these presentations [28,34–36].
Nervous System Manifestations
Neuroborreliosis is a common complication of European and North American-acquired Lyme borreliosis, mainly presenting within a few weeks to months of infection, in about 10% and 20% of previously untreated patients, often with concurrent or recent erythema migrans. It can affect both peripheral and central nervous systems. The most common manifestation in children is isolated facial nerve palsy without clinical signs of meningitis, although CSF examinations of some patients show mononuclear pleiocytosis [37,38]. It has been postulated that the higher rate of tick bites occurring on the head and neck region of young children causes a relatively higher incidence of facial palsies in children than in adults [39]. Other neurological presentations in children include ‘viral-like’ meningitis with or without facial or other cranial nerve palsies, including sixth nerve palsy [37,38]. Rare cases have been reported of children with previously untreated Lyme neuroborreliosis presenting with raised intracranial pressure and very high CSF protein levels, leading to sight-threatening complications [40]. Other uncommon complications include meningoencephalitis and encephalopathy.
Garin–Bujadoux–Bannwarth (GBB) syndrome is the most common neurological complication in adults. Features include facial palsy, meningitis and radiculoneuritis, usually presenting as a painful radiculopathy similar to that of shingles or mechanical radiculopathy. It was originally described by French physicians in 1922 and a German neurologist in 1941 [41,42]. A more indolent form of radiculopathy has been recognized in some European patients, mainly in older age groups, who present with a gradual onset of pain increasing over months after infection acquisition. This may result from direct spirochaetal invasion of peripheral nerves from the initial skin inoculation site, gradually extending to the nerve roots, in contrast to the more rapid onset and wider effects seen in classic GBB syndrome, resulting from haematogenous spread [13]. In both situations radicular pain can be severe and patients may require opiates, but analgesia requirements usually reduce very significantly within a short time of commencing antibiotic treatment. Painful radiculopathy is rare in children.
Meningoencephalitis is an uncommon complication in both children and adults. Encephalomyelitis is rare, mainly seen in older adults. It appears to affect white matter disproportionately and may be confused with multiple sclerosis or, if particularly acute, with acute disseminated encephalomyelitis. Virtually all patients have an inflammatory CSF and intrathecal production of B. burgdorferi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree