A haemostatic imbalance occurs following the activation of coagulation and dysfunction or dysregulation of the natural regulatory pathways (Fig. 55.2) [48,50]. Endothelial prostacyclin production and the expression of anticoagulant glycosaminoglycans may be impaired [55,56]. Patients with meningococcal disease have increased monocyte tissue factor activity [57], decreased tissue factor pathway inhibitor [58], a deficiency of antithrombin, protein C and protein S, and significant disruption of the fibrinolytic pathway [45,54,59–64]. We have demonstrated that disruption of the endothelial activation of protein C in meningococcal sepsis is caused by downregulation of thrombomodulin and endothelial protein C receptor on the endothelial cell surface (Figs 55.3, 55.4) [54].
Fig. 55.2 The coagulation and fibronolytic pathways rely on the function of endothelial proteins and protein complexes. Dysfunctional regulatory mechanisms in meningococcal disease include endothelial (protein C pathway) and plasma (tissue factor pathway inhibitor (TFPI), antithrombin, PAI) factors. PGI2, prostacyclin; TFPI, tissue factor pathway inhibitor; AT, antithrombin; GAGs, glycosaminoglycans; CS, chondroitin sulphate; HS, heparin sulphate; DS, dermatan sulphate; PC, protein C; PS, protein S; TM, thrombomodulin; EPCR, endothelial protein C receptor; PAI, plasminogen receptor inhibitor; tPA, tissue plasminogen activator.
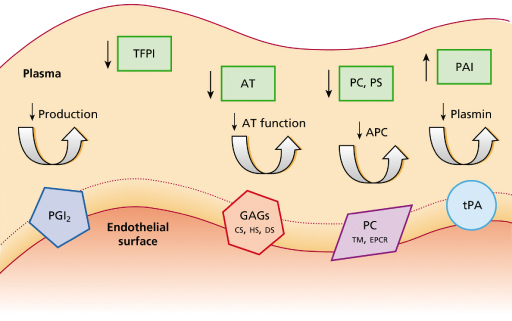
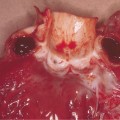
Fig. 55.3 Skin-biopsy specimen from a patient with meningococcal sepsis. A biopsy specimen from a purpuric lesion shows areas containing thrombosed vessels (right-hand arrow in (a) and arrow in (b)) and a perivascular infiltrate (left-hand arrow in (a)) (haematoxylin and eosin, ×100 (a) and ×400 (b)). (c) shows inflammatory cells (arrow) around non-thrombosed vessels (haematoxylin and eosin, ×400). The cellular infiltrate consisted of both neutrophils (identified by neutrophil-elastase staining) and monocytes and macrophages (identified by CD68 staining).
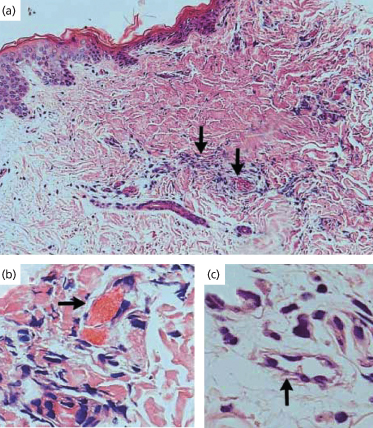
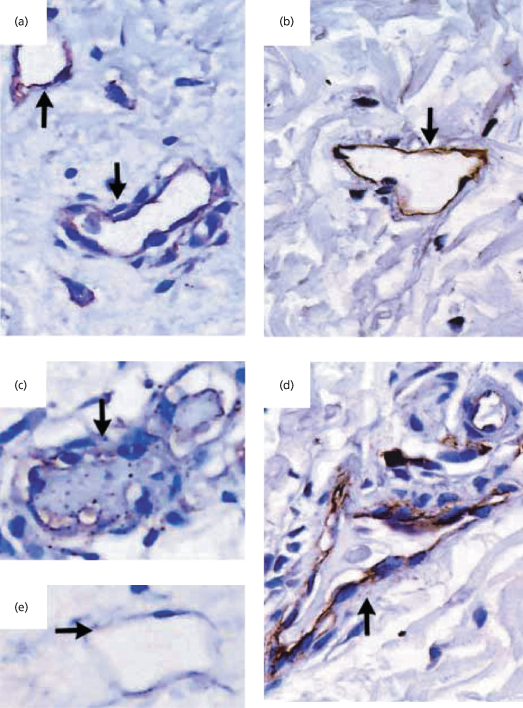
Fig. 55.4 Immunostaining for thrombomodulin in skin biopsy specimens from patients with meningococcal sepsis during the initial infection and 3 months later (immunoperoxidase stain, ×200). Thrombomodulin immunostaining of skin biopsy specimens from a patient with acute meningococcal sepsis during the initial infection (a) and 3 months later (b) is shown. The same sequence is shown for a second patient (c, d). (e) shows another initial specimen from the second patient. The arrows in (a) and (e) show unthrombosed vessels with reduced thrombomodulin staining, and the arrow in (c) shows a thrombosed vessel with reduced thrombomodulin staining. Partial recovery of thrombomodulin expression (arrows in (b) and (d)), together with some residual inflammatory infiltrate, is seen in both patients after 3 months.
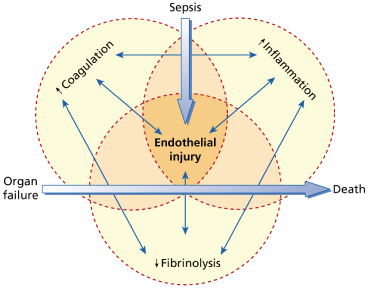
The dysregulation of the coagulation and fibrinolysis pathways described above occurs together with activation of inflammatory pathways including innate, cell-mediated and humoral immunological processes, involving the complement cascade, the kallikrein-bradykinin system, monocytes, neutrophils and cytokines (Fig. 55.5) [48,50,65–69]. The pathways are linked at many levels [48], together leading to the capillary leak, defects in vascular tone, hypoperfusion, myocardial dysfunction and focal thrombosis that are responsible for the tissue damage commonly observed in association with fulminant meningococcal sepsis [48].
Fig. 55.5 The inflammatory, coagulation and fibrinolytic pathways are linked at many levels, leading to organ failure and eventually death.
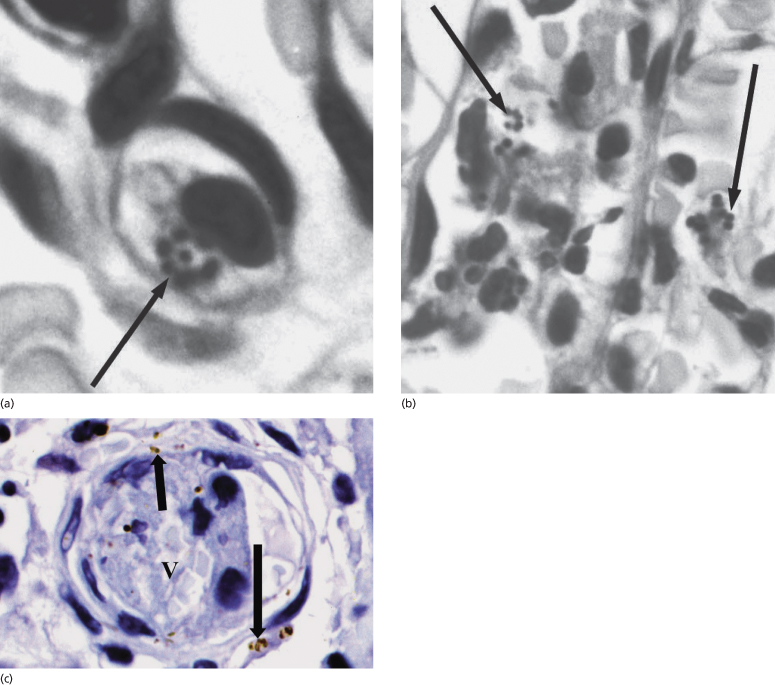
Although the release of meningococcal LPS seems to be an important trigger for the inflammatory and haemostatic processes seen in fulminant disease, the importance of direct invasion of the skin by the organism remains uncertain. However, in view of the large numbers of meningococci detected in the dermal vessels of purpuric skin lesions [36,39,42,70], direct involvement by the organism is likely. There is increasing evidence that meningococci adhere to the endothelium through a number of mechanisms, and may not only be directly toxic but act as a focus for both the inflammatory and haemostatic responses on the vessel wall [69,71–73]. We have identified meningococci in a number of different cellular environments expressing key virulence factors (Fig. 55.6) [70].
Fig. 55.6 Skin biopsy specimen from a patient with meningococcal septicaemia. (a, b) Gram-stained sections reveal meningococci associated with a leucocyte (a, electronically enlarged) and within blood vessels (b, electronically enlarged). Figures were processed with Adobe Photoshop and illustrator software. Arrows indicate Gram-stained meningococci. (c) Immunohistochemical staining of N. meningitidis in a skin biopsy sample from a patient with meningococcal disease showing PorA-stained bacteria in the interstitium and blood vessel. V, blood vessel. Arrows indicate immunoperoxidase-positive staining of meningococci (brown) with the appropriate specific mouse monoclonal antimeningococcal antibody (nuclei were counterstained in haematoxylin).
While many single-gene defects are known to affect the immune and coagulation responses to infection [74], a combination of genetic predisposition and bacterial virulence factors is likely to be responsible for any one individual’s susceptibility to invasive disease. In meningococcal disease, many such single-nucleotide polymorphisms have now been identified. Genetic variants of mannose-binding lectin might account for nearly one-third of cases of invasive disease [75] and an innate anti-inflammatory cytokine profile contributes to the likelihood of death [76]. A specific Fc-γ receptor polymorphism may be essential in protecting against fulminant meningococcal infection [77]. Although the factor V Leiden mutation confers a specific genetic predisposition to thrombotic disease [78], it has not been shown to be associated with increased mortality in meningococcaemia, although patients with this mutation may be at higher risk of thrombotic complications such as amputations and skin grafting [79]. An increased PAI-1 response to TNF-α has been associated with death in meningococcal septicaemia [80], demonstrated to be due to a polymorphism in the PAI-1 gene [63,64].
Clinical Features.
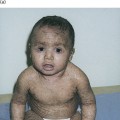
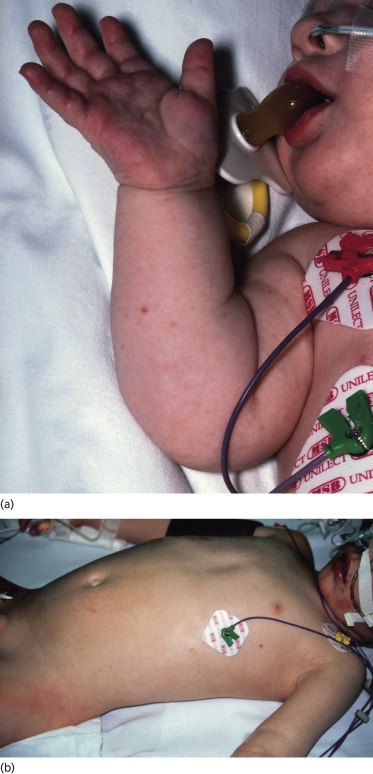
Meningococcal meningitis is the most common form of the disease, is indistinguishable from other forms of childhood bacterial meningitis, usually responds rapidly to antibiotics and is associated with little long-term morbidity [81]. Meningococcal septicaemia with shock is more devastating, commonly presenting with non-specific symptoms of fever, vomiting, headache, abdominal pain and muscle aches. The rash of meningococcal septicaemia may be very subtle in the early stages (Fig. 55.7) and its recognition is imperative if this life-threatening condition is to be diagnosed early and treated effectively.
Fig. 55.7 Early stages of meningococcal septicaemia. (a) Fine maculopapular rash with one early purpuric lesion on the forearm of a young infant. (b) Fine maculopapular rash with a few early purpuric lesions on the trunk of a young child with septic shock.
In the early stages of meningococcal infection the rash may resemble a viral exanthem such as rubella (see Fig. 55.7). This erythematous maculopapular rash is non-purpuric, non-pruritic and often very transient [36,82]. Following a diligent search of all areas of the body, the purpuric lesions of meningococcal septicaemia are seen in 80–90% of patients [83]. The rash usually occurs within 12–36 hours of the onset of the disease and is characteristically petechial but may blanch early in the course of the infection. In the classic presentation of meningococcal septicaemia, the petechiae are irregular, small and often have raised centres (Fig. 55.8). Lesions commonly occur on the limbs and trunk but may be found on the head, palms, soles and mucous membranes. They may also occur on areas subjected to friction or under pressure by clothing.
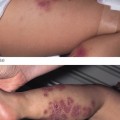
Fig. 55.8 Purpuric lesions of meningococcal sepsis. (a) Purpuric lesions on trunk and limbs. (b) Close-up of abdominal purpuric lesions. (c) Haemorrhagic rash with central necrosis. (d) Severe purpura fulminans. (e) Bullous formation on a large ecchymotic area.
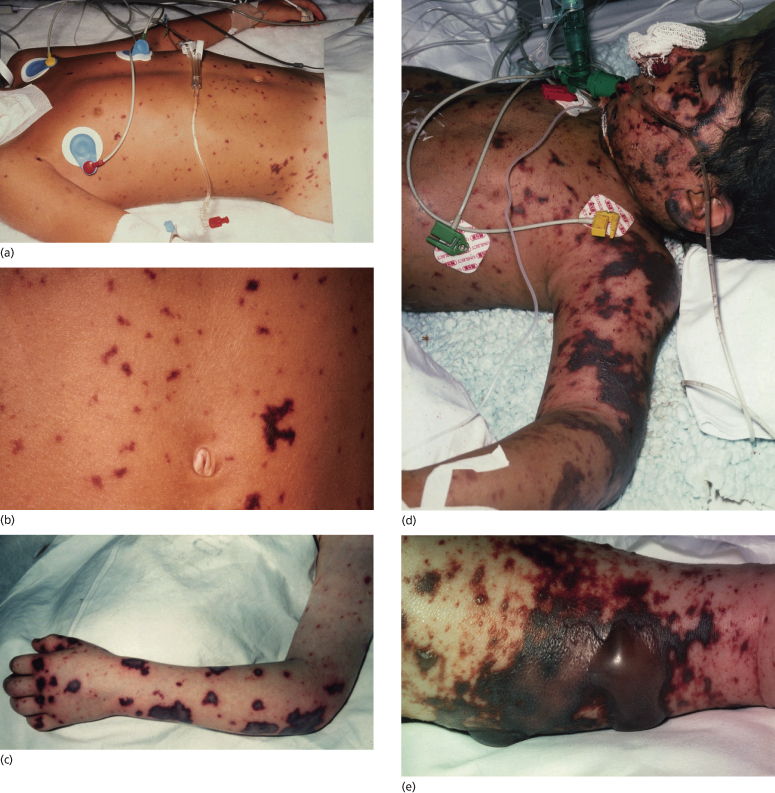
Progression of the rash of meningococcal septicaemia has been shown to relate to the severity of the coagulopathy and prognosis [41,49,84–87]. As the disease becomes more fulminant, lesions coalesce to form large ecchymoses and become haemorrhagic and bullous (see Fig. 55.8). These areas of extensive skin involvement may progress to gangrene of the digits or whole limbs (Fig. 55.9). In such severe purpura fulminans, the gangrenous regions are gunmetal grey or blue-black and are well demarcated [37]. The arterial pulses are usually intact but the capillary perfusion is very poor. Thrombosis, extensive skin involvement, venous congestion and tissue oedema may lead to the development of a limb compartment syndrome (see Fig. 55.9). Severe tissue destruction may be associated with muscle infarction and rhabdomyolysis.
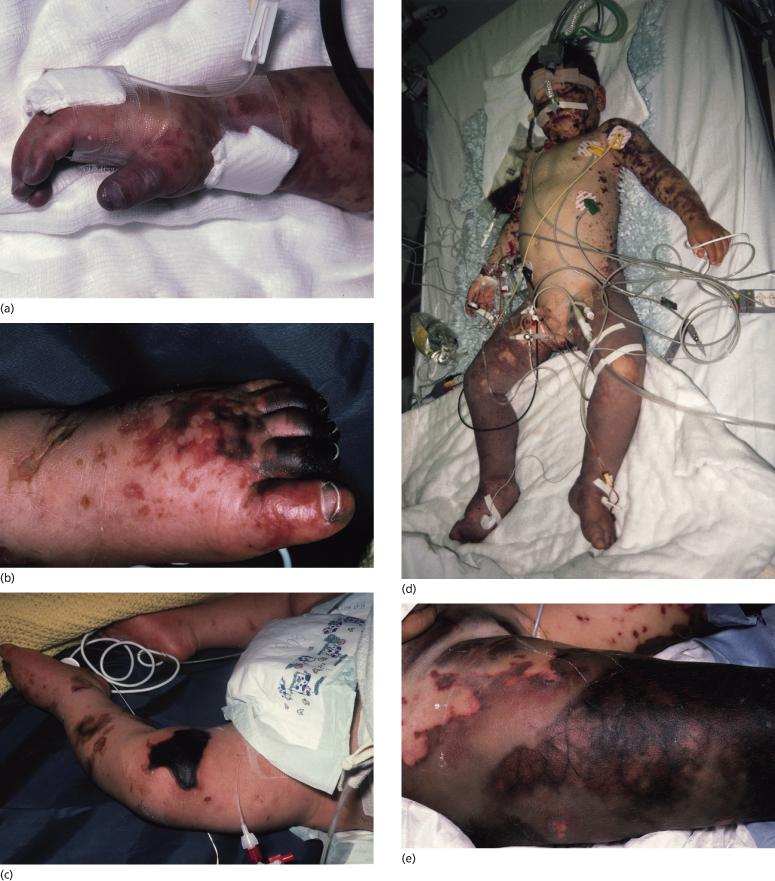
Fig. 55.9 Extensive cutaneous involvement in meningococcal sepsis. (a) Severe digital hypoperfusion in the early stages of meningococcal septicaemia. (b) Digital hypoperfusion that has progressed to gangrene with demarcation 2 weeks after onset of the illness. (c) Gangrene over a proximal ecchymotic area associated with dermal vascular thrombosis. (d) A child with septicaemic shock in the first 24 h of the illness, showing purpura fulminans, severe bilateral lower limb ischaemia and bilateral compartment syndromes. (e) Extensive gangrene with marked venous thrombosis of superficial and deep vessels.
The development of shock associated with meningococcal septicaemia may be insidious, and is manifested initially by poor peripheral perfusion, confusion and an increased respiratory rate. Hypotension is a late sign as children and young adults may maintain their blood pressure by vasoconstriction, despite severe hypovolaemia [3]. It is important to recognize meningococcal shock in the early phases, well before hypoxia, acidosis and hypovolaemia lead to multiorgan failure (Fig. 55.10). An algorithm for the early recognition and management of meningococcal infections is given in Figure 55.11.
Fig. 55.10 Severe meningococcal sepsis associated with a capillary leak syndrome and multiorgan failure. This child had widespread purpuric lesions, poor peripheral perfusion and marked tissue oedema and required mechanical ventilation, inotropic support and peritoneal dialysis.
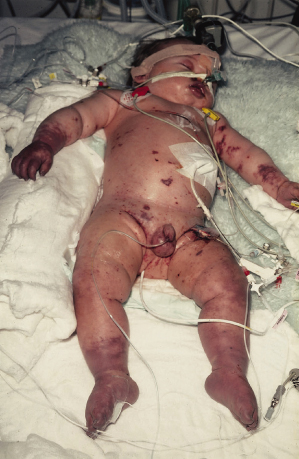
Fig. 55.11 Early management of meningococcal disease in children.
Reproduced with permission from the Meningitis Research Foundation. © Meningitis Research Foundation (www.meningitis.org).

Differential Diagnosis.
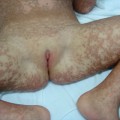
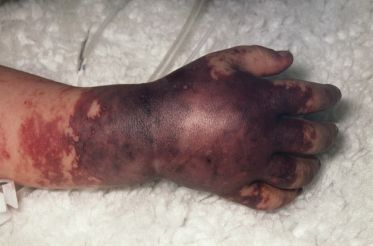
Bacteraemia with Haemophilus influenzae, Streptococcus pneumoniae, Pseudomonas spp. and overwhelming viral and fungal infections may all rarely present with shock and purpuric rash (Fig. 55.12). Other congenital and immune causes of a purpura fulminans may present with the characteristic rash but these patients are rarely cardiovascularly compromised (see Chapter 162).
Fig. 55.12 Differential diagnosis of meningococcal sepsis. The hand of a child with a Streptococcus pneumoniae septicaemia. Although this presentation is rare, the confluent purpuric lesions, tissue oedema and compartment syndrome are very similar to those seen in meningococcal septicaemia.
Laboratory Diagnosis.
Meningococci are very sensitive to chilling or drying, and must be inoculated and cultured soon after collection. Cerebrospinal fluid (CSF) examination and culture are likely to provide confirmation of the diagnosis of meningococcal meningitis in about 80–90% of cases [88]. However, blood cultures are positive in only one-third to one-half of untreated patients, even when accompanied by a characteristic rash or positive CSF microscopy and culture [88]. Lumbar puncture is now usually considered contraindicated when the clinical diagnosis is meningococcal septicaemia or is often delayed when a characteristic rash suggests meningococcal meningitis, as it is frequently associated with clinical deterioration and death [3,89]. Nasopharyngeal swabs may provide evidence of meningococcal infection. Biopsy material and aspirates from the characteristic purpuric skin lesions of meningococcal disease provide a rich source of organisms and have been advocated by some workers for rapid diagnosis, but this is not usually considered routine owing to the advent of modern molecular techniques now available [88,90–94].
An array of rapid tests is now available to detect N. meningitidis group A, B, C, Y and W135 antigens from blood, CSF and urine, which may establish the diagnosis although the sensitivity is poor [95,96]. The highly sensitive polymerase chain reaction (PCR) of blood (or CSF where available) can make the diagnosis in an additional 60% of cases in which no positive culture has been obtained [97–100]. The sensitivity of the new WB-Taqman PCR on EDTA-treated samples of whole blood has been demonstrated to be 87%, increasing case confirmation compared with previous PCR techniques from 72% to 94% [100].
Management of Acute Meningococcal Septicaemia.
The management of meningococcal disease has been reviewed in detail elsewhere [3,83] and is detailed in Figure 55.11. However, a number of important management points will be emphasized.
Recognition and Initial Therapy
It is crucial that, in the absence of another diagnosis, any febrile, ill child with a petechial rash should be considered to have meningococcal septicaemia and treatment should be commenced without awaiting further confirmation. Although the majority of children may not appear desperately ill when first seen, they may deteriorate suddenly and therefore should be observed carefully during the first 48 hours, ideally on a paediatric intensive care unit.
Choice of Antibiotic
Penicillin remains the treatment of choice for meningococcal sepsis. However, since other infections may also occasionally cause shock and a petechial rash, a third-generation cephalosporin should be employed until the diagnosis has been confirmed. Penicillin-resistant strains of N. meningitidis have become a problem in Spain and South Africa [101–103] but fortunately only a handful of insensitive strains have been detected in the UK, the rest of Europe and North America [104–111].
Supportive Care
Children who present in meningococcal shock need immediate resuscitation, restoration of circulating volume and adequate oxygenation [3]. In those patients with concomitant meningitis, cerebral oedema and raised intracranial pressure may occur. Elective ventilation, control of the PCO2, improvement of cardiac output and thus cerebral perfusion are important aspects of treatment of such patients.
Management of the Skin Lesions
Although the majority of purpuric skin lesions heal well following the resolution of meningococcal sepsis, in fulminant disease it is important to prevent the extensive skin damage, limb loss and associated multiorgan failure that often occur despite prompt antibiotic therapy.
Although there is no evidence from randomized controlled trials, fresh frozen plasma (FFP) infusions are indicated to correct hypofibrinogenaemia and specific clotting factor deficiency, and to reduce the risk of haemorrhage [50,112]. Cryoprecipitate may be used in patients where hypofibrinigenaemia persists despite multiple FFP infusions. In addition, prostacyclin has been used to try to reverse some vasoconstriction where impending gangrene is seen in a ‘glove and stocking’ distribution. However, there is no clinical trial supporting its use and, when used in large doses, this agent may cause further hypotension [50,55].
Faced with a devastating illness, and the prospect of limb loss and death in young children, many clinicians have felt justified in attempting to utilize experimental treatments outside controlled trials. Purified inhibitors such as antithrombin, fibronectin, protein C or variants of α1-antitrypsin have been advocated by a number of authors [59,113–120], as have blockers of the initial phases of coagulation [121–123]. Others have suggested fibrinolytic agents [124]. Heparin has been promoted in the past as rational therapy for disseminated intravascular coagulation DIC [39,125,126]. However, the agent has failed to produce obvious benefit in a limited number of open clinical trials [40,127,128] and has been associated with uncontrolled bleeding.
In recent years, understanding of the pathophysiology of the coagulation and inflammatory abnormalities has led to a number of large randomized controlled trials of adjunctive agents in adult and childhood sepsis and in meningococcal disease. No survival or morbidity benefit has been demonstrated for most of these agents, including antithrombin [129], antiendotoxin monoclonal antibody [130] and recombinant bactericidal permeability-increasing protein [131]. Although previous trials of steroids in sepsis have been disappointing [132,133] and a very old study suggested a worse prognosis in meningococcaemia [134], more recent data have renewed interest in low-dose steroid therapy [135]. A phase III trial of recombinant activated protein C was shown to reduce the mortality in adult sepsis [136], although a recently completed phase III trial in childhood sepsis did not show similar benefit [137]. On the basis of small pharmacological series using unactivated protein C concentrate, some have advocated using this form of the drug [119,120,138]. However, it is currently not possible to predict which patients who have been administered protein C concentrate will be able to activate the drug [54,138]. Even when activated protein C is detectable in the plasma, there is evidence of an activation defect in the dermal vascular endothelium and a theoretical risk that unactivated protein C in excess may displace activated protein C at the endothelial protein C receptor and thus worsen dermal vessel thrombosis [54,139].
Finally, although the use of recombinant tPA to treat severe meningococcaemia has an apparently sound theoretical basis [61,63,64,80], in a retrospective European study of 62 children with meningococcal disease treated with tPA, 8% suffered serious intracerebral bleeding and its use cannot now be recommended [140]. The adverse clinical experience demonstrated in evaluating tPA and the lack of benefit of most of the other adjunctive therapies used to treat severe sepsis strongly reinforce the need for new or experimental agents to be introduced only after properly controlled randomized clinical trials.
Surgical Intervention
In the acute phase of fulminant meningococcaemia, surgical intervention has been attempted in the authors’ units to relieve compartment syndromes that may develop in severely affected limbs [141] (Fig. 55.13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree