R, reservoir hosts.
Poxviruses are large complex DNA viruses which replicate in the cytoplasm of infected cells using virus-associated, DNA-dependent polymerase and are characterized by the production of skin lesions. All poxviruses encode cytokine and cytokine receptor analogues which allow them to modulate the immune response and perpetuate their existence. They are not only cytopathic, causing host tissue damage, but also encode an array of immunomodulatory molecules that affect the severity of disease [3]. They are generally brick shaped or ovoid, varying in size from 240 × 300 nm (orthopoxvirus) to 160 × 250 nm (parapoxvirus) [1,2].
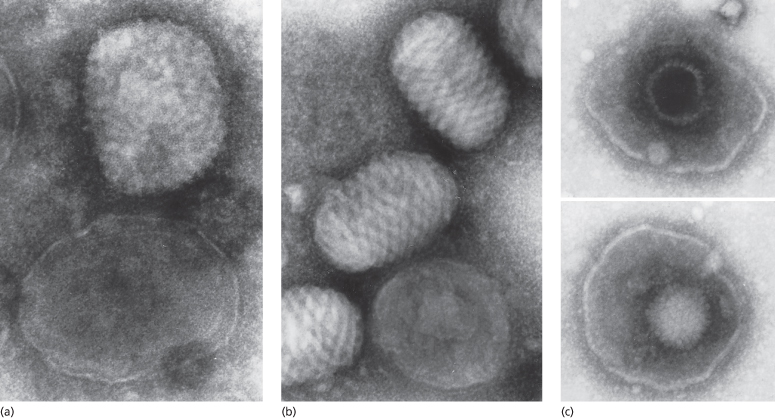
Electron microscopy is of considerable value in rapid diagnosis [4,5] (Fig. 51.1). Their size and the presence of conspicuous surface tubules enable poxviruses to be differentiated from herpesviruses. Furthermore, the characteristic long, spirally wound, surface tubule of the parapoxviruses enables them to be differentiated from orthopoxviruses and tanapoxviruses. The epidemiological history, clinical features and electron microscopy will in many instances indicate a certain diagnosis but if necessary, the viruses may be isolated and identified [2,4]. Rapid identification by orthopoxvirus-specific polymerase chain reaction (PCR) is available in some centres [6–9]. Specialized laboratories are available for the diagnosis of smallpox should the need arise.
Fig. 51.1 Electron micrographs of (a) cow poxviruses showing the common ‘mulberry’ (M) form with conspicuous short randomly arranged surface tubules and the less common ‘capsule’ (C) form with deeper stain penetration. (b) Whole M and C forms of parapoxvirus, showing the characteristic long spiralling of the surface tubule of the former. (c) Whole herpesviruses showing one particle with intact nucleocapsid and one broken, allowing stain penetration (phosphotungstic acid, ×120,000)
Reproduced with permission from Baxby D, Bennett M. Cowpox: a re-evaluation of the risks of human cowpox based on new epidemiological information. In: Kaaden OP, Czerny CP, Eichorn W. (eds) Viral Zoonoses and Food of Animal Origin. Vienna: Springer, 1997: 1–12.
Parapoxviruses are not routinely isolated by the diagnostic laboratory. A differential diagnosis of orf or milker’s nodules is based on animal contact if known; otherwise a diagnosis of orf/milker’s nodules is often reported. Poxviruses are resistant to environmental conditions and will survive sufficiently in dried scabs or in vesicle fluid dried on a microscope slide to enable them to be transported by first-class post to the laboratory. However, if a differential laboratory diagnosis is required, virus transport medium should be used to preserve more labile viruses. Zoonotic poxviruses will grow well in human cell cultures and in cells from their reservoir hosts; orthopoxviruses grow well on chick chorio-allantois [4,5]. Preliminary identification is by suitable biological markers, but increasing use is being made of DNA analysis [6–11], which enables individual strains within a species to be recognized [7,11]. A review on human zoonotic poxvirus infections focuses on recent publications, including newer therapeutic agents [12].
References
1 Esposito JJ, Baxby D, Black DN et al. Poxviridae. In: Murphy FA, Faquet CM, Bishop DHL (eds) Virus Taxonomy: Classification and Nomenclature of Viruses. Vienna: Springer-Verlag, 1995: 79–91.
2 Baxby D. Poxviruses. In: Mahy BWJ, Collier LH (eds) Topley and Wilson’s Microbiology and Microbial Infections, vol. 1, 9th edn. London: Arnold, 1998: 367–83.
3 Stanford MM, McFadden G, Karupiah G, Chaudhri G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol Cell Biol 2007;85(2):93–102.
4 Nakano JH, Esposito JJ. Poxviruses. In: Schmidt NJ, Emmons RW (eds) Diagnostic Procedures for Viral, Rickettsial and Chlamydial Diseases, 6th edn. Washington, DC: American Public Health Association, 1989: 224–65.
5 Baxby D, Bennett M, Getty B. Human cowpox 1969–93: a review based on 54 cases. Br J Dermatol 1994;113:598–607.
6 Ropp SL, Jin Q, Knight JC et al. PCR strategy for identification and differentiation of smallpox and other orthopoxviruses. J Clin Microbiol 1995;33:2069–76.
7 Naidoo J, Baxby D, Bennett M et al. Characterisation of orthopoxviruses isolated from feline infections in Britain. Arch Virol 1992;125:261–72.
8 Loparev VN, Massung RF, Esposito JJ et al. Detection and differentiation of old world orthopoxviruses: restriction fragment length polymorphism of the crmB gene region. J Clin Microbiol 2001;39:94–100.
9 Lapa S, Mikheev M, Shelkunov S et al. Species-level identification of orthopoxviruses with an oligonucleotide microchip. J Clin Microbiol 2002;40:753–7.
10 Espy MJ, Cockerill FR III, Meyer RF et al. Detection of smallpox virus DNA by LightCycler PCR. J Clin Microbiol 2002;40:1985–8.
11 Inoshima Y, Morooka A, Sentsui H. Detection and diagnosis of parapoxvirus by the polymerase chain reaction. J Virol Methods 2002;84:201–8.
12 Lewis-Jones MS. Zoonotic pox virus infections in man: review article. Curr Opin Infect Dis 2004;17:81–9.
Orthopox Infection in Humans
Smallpox
Historical Aspects.
Smallpox (variola major) has altered the course of world history [1,2]. For centuries an endemic disease throughout the world, it caused millions of deaths and frequently left survivors with damaged health, unsightly pock-marked skin and blindness. In mediaeval times, the overall mortality ranged from 10% to 60%, with an average of about 25–30%. By the 20th century, mortality had fallen to between 5% and 25%, although the childhood mortality rate remained as high as 40% [2,3].
The origins of smallpox are uncertain. During the first millennium AD, the disease spread through Asia into Europe and, around 700 AD, into North Africa [1–3]. The Spanish Conquistadors carried it to the New World in the 1500s, where it spread rapidly in the ‘virgin population’. It is estimated that over 3.5 million native South Americans died in smallpox epidemics. A similar epidemic occurred in North America when smallpox wiped out millions of the members of native tribes [1–4]. The annual smallpox mortality rate in Europe in the 18th century was estimated at about 200,000–600,000, making up 7–12% of all deaths. The majority of these mortalities occurred in children, especially infants, and the disease accounted for one-third of all childhood deaths [1]. The succession of the European monarchy was considerably altered by smallpox deaths [1].
In the early 20th century, a minor form of smallpox, alastrim, with a mortality of 1% or less, appeared in Africa and spread throughout the USA and Europe [3–6].
Vaccination alone was unsuccessful in eradicating smallpox and in 1967, when the estimated world prevalence was 10–15 million, the World Health Organization (WHO) commenced a global programme of identification, isolation and containment of cases and contacts [2,3]. As a result, the last naturally occurring case of smallpox was recorded in Somalia in 1977, although a small UK laboratory outbreak did occur in 1978 [3]. In 1980, the WHO declared the world to be free from smallpox, the first and to date the only virus infection to have been successfully eradicated [2,3]. After this, all stocks of smallpox virus were destroyed except for those held in specialized laboratories in the USA and the former USSR for research purposes [4].
Aetiology.
The smallpox virus is a large brick-shaped orthopox virus measuring 240 × 300 nm (Fig. 51.1a). The virus is very stable and in the past attempts to attenuate it proved largely unsuccessful [3,7]. Epidemics were most common in winter and spring, transmission occurring by inhalation of infected droplets in aerosolized form or indirectly from fomites in clothes and bedding [2–6]. The infectious dose is unknown but is likely to be very small [4,8]. There are no known animal or insect vectors [2–4].
The virus enters through the mucosal surfaces of the nasopharyngeal tract and is transmitted to the local lymph nodes, where it replicates. After 3 or 4 days, an asymptomatic viraemia occurs, transmitting the smallpox virus to the rest of the reticuloendothelial system. Further replication occurs in the spleen, bone marrow and lymph nodes. Between 8 and 10 days after exposure, infected leucocytes cause a second symptomatic viraemia, homing particularly to the vessels in the papillary dermis and infecting local epidermal cells to produce cutaneous lesions. Persons incubating the disease become infectious only at this point. Their saliva is most infectious during the first week of illness and especially when oral lesions rupture. Ruptured pustules are also very infectious, but scabs much less so [2–4]. The virus is not as contagious as chickenpox or influenza and the secondary attack rate among unvaccinated contacts ranges from 37% to 88% [3]. Transmission usually occurs among close contacts, but occasionally there are more extensive outbreaks [8].
Clinical Features.
The most recent contemporary illustrated accounts of the clinical features of smallpox are found in publications by Dixon in 1962 [6], Rao in 1972 [9] and Fenner et al. in 1988 [3]. Rao [9] studied 3544 cases in India and reclassified smallpox into five types (Table 51.2). This classification was later adopted by the WHO [3].
1 ‘Ordinary’ smallpox accounted for the majority of cases: 88.8% of unvaccinated patients and 70% of vaccinated patients. There are three subtypes. The confluent subtype was the most serious, with a mortality of 62% in the unvaccinated, and was characterized by lesions that were confluent on the face and forearms but discrete elsewhere. Patients with the semi-confluent subtype had confluent lesions only on the face (37% mortality), while those with the discrete form, the most common type, had no confluent lesions (9.3% mortality).
2 A milder, modified type, rarely fatal and with an accelerated course, occurred in 2% of unvaccinated persons and 25% of those previously vaccinated.
3 The rare purpuric or haemorrhagic-type smallpox (3%, usually adults), with widespread haemorrhage into skin and mucous membranes, caused early death from septicaemia. Subconjunctival haemorrhages and bleeding from any orifice could occur. Characteristically, the incubation period was short and the prodromal illness was severe and accompanied by abdominal pain. The ‘early type’ was always fatal and the ‘later’, with haemorrhage into pustules, usually fatal. It occurred more commonly in pregnancy.
4 The flat type (7%), fatal in 66% of cases, occurred mainly in children (72%). A short incubation period and severe prostrating illness was followed by confluent or semi-confluent, slowly developing lesions. These lesions never formed pustules but remained soft and flattened with an erythematous and velvety appearance. The distribution was not always centrifugal. In survivors, the skin healed without scab formation and widespread epidermal peeling sometimes occurred.
5 In variola sine eruptione, fever occurred without rash in previously vaccinated persons or in infants with maternal antibodies. This form of smallpox could only be confirmed by serological testing. This last type may not cause transmissible smallpox.
Table 51.2 A classification of clinical types of variola major
Reproduced from Fenner et al. 1988 [3] (based on Rao 1972 [9]) published with permission of the World Health Organization.
| Type | Features |
| Ordinary type | Raised pustular skin lesions
|
| Modified type | Like ordinary type but with an accelerated course |
| Variola sine eruptione | Fever without rash caused by variola virus; serological confirmation |
| Flat type | Pustules remained flat; usually confluent or semi-confluent; usually fatal |
| Haemorrhagic type | Widespread haemorrhages in skin and mucous membranes
|
The incubation period for ‘ordinary’ smallpox is around 10–14 days (range 7–17 days) [2–6]. Patients are largely asymptomatic and remain non-infectious until the end of the second week, when there is an abrupt onset of a high prodromal fever associated with severe malaise and ‘flu-like’ symptoms. Most have severe headache, usually frontal, and backache is common. Vomiting occurs in about half of patients, sometimes with severe abdominal pain. About 10% of patients, especially infants, develop diarrhoea. Very rarely, infants develop acute dilation of the stomach, which is usually fatal. Some adults become delirious and convulsions may occur in children. The fever falls by the second or third day and erythematous mucosal lesions (enanthema) appear in the mouth and pharynx, often associated with pharyngitis. The exanthema develops the following day and consists of small cutaneous erythematous macules [3–6,9].
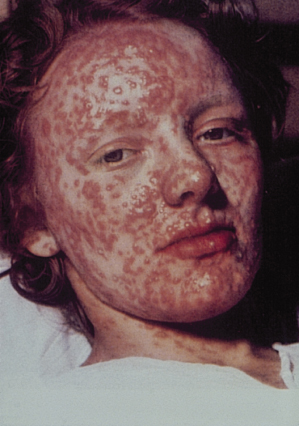
The eruption is characteristically centrifugal, developing initially on the face, hands and palms and then spreading distally to the trunk and limbs and the soles (Figs 51.2–51.4). The lesions evolve more slowly than in chickenpox, developing over a period of 4–7 days. The lesions begin as small ‘papules’, in reality early vesicles, which are firm and hard. They evolve briefly into 2–4 mm vesicles and then into deep, firm, circular pustules (4–6 mm). In severe cases, these lesions become confluent. At any one time, unlike chickenpox, all lesions are in the same stage of evolution. By the seventh or eighth day of the rash, the pustules start to flatten and become umbilicated (Fig. 51.3) and then gradually crust by the end of the second week, lasting longest on the palms and soles. Final resolution of lesions occurs at about the end of the third week of the rash, leaving postinflammatory hyper- and/or hypopigmentation initially. Although this gradually fades, a proportion develop fibrosis, causing deeply pitted scars or ‘pock’ marks of 2 mm or more. Destruction of sebaceous glands causes the face to be particularly affected in 65–80% (Fig. 51.5) [3–6]. Involvement of organs other than the skin, mucous membranes and reticuloendothelial system is uncommon and most deaths (usually between days 10 and 16) result from toxaemia and hypotension, probably caused by circulating immune complexes and soluble variola antigens [3,4]. Smallpox infection confers lifelong immunity in most cases, but occasional subclinical smallpox was reported in patients who had previously had smallpox [3].
Fig. 51.2 (a–c) ‘Ordinary’ smallpox, discrete type, demonstrating the centrifugal distribution of lesions, particularly on the face and distal limbs, hands and feet with fewer lesions on the trunk.
Reproduced from the Smallpox Eradication Campaign identification pamphlet, with permission from the World Health Organization.
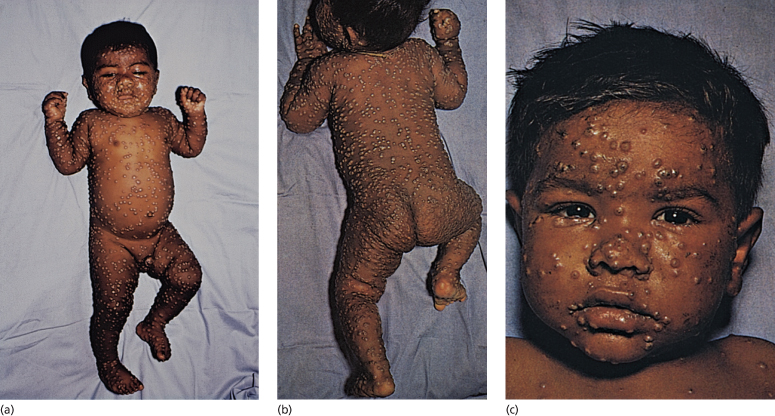
Fig. 51.3 Close-up of typical lesions on the hands and feet showing involvement of the palms and soles with early umbilication of lesions in (a).
Reproduced with permission from the World Health Organization.
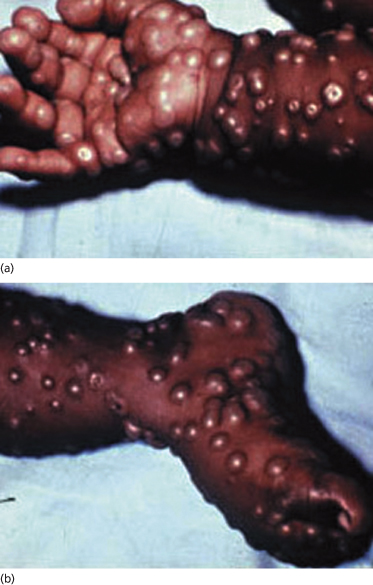
Fig. 51.4 ‘Ordinary’ smallpox in a child at the umbilicated/crusted stage. Note the confluent lesions on the face.
Courtesy of Dr Derrick Baxby.
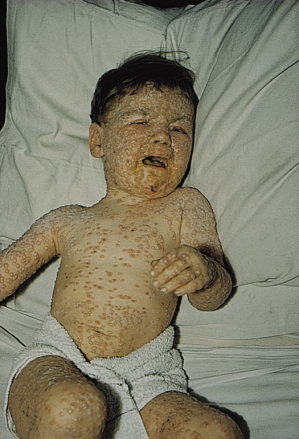
Fig. 51.5 Granulomatous smallpox lesions of the face causing destruction of the sebaceous glands.
Reproduced from Dixon [6] with permission from Elsevier.
The fever of ordinary-type smallpox is bimodal. In the prodromal stage, there is a peak of around 40°C, followed by low-grade pyrexia for a few days. The second peak of around 39°C occurs during the maximum evolutional or pustular stage in the second week of the rash and gradually subsides, often in a swinging pattern [3–6]. Persistence of fever after scab formation is a poor prognostic indicator [3]. Respiratory involvement and cough, with death from pneumonia, is more likely to occur in severe cases. Blindness was reported as common in the older literature but occurred in only about 1% of patients in the 20th century [3,6,10]. It was usually as a result of keratitis, corneal ulceration and scarring and rarely secondary infection or panophthalmitis. Encephalitis was reported in 1 in 500 cases of variola major and 1 in 2000 of variola minor [3]. It usually resolved slowly without complications, but very rarely (1%) a typical perivascular demyelination developed, similar to that occurring with vaccinia, measles or varicella [6]. Arthritis and osteomyelitis developed in about 2% of children, possibly as a result of viral involvement of the metaphyses [3,10]. Signs may be missed in the acute stages, but later sequelae of limb shortening, flail joints, subluxations and gross deformities may occur [3]. As variola is cytocidal, most pregnant women miscarry in the early stages of smallpox; however, if the fetus survives to term it has temporary immunity from maternal antibodies [3]. Congenital smallpox is usually fatal [3,9].
In a few vaccinated patients, there may be a fleeting erythematous rash during the prodromal stage, particularly around the vaccination scar and in the axillae, groins and popliteal fossae [3,4]. The more toxic forms of smallpox, apart from the haemorrhagic type, are less common in vaccinated individuals [3].
‘Modified-type’ smallpox sometimes occurs in previously vaccinated individuals and is hardly ever fatal. The prodrome may still be severe but the course of the disease is accelerated, the lesions often being smaller and fewer and the secondary eruptive fever absent [3,9].
Differential Diagnosis.
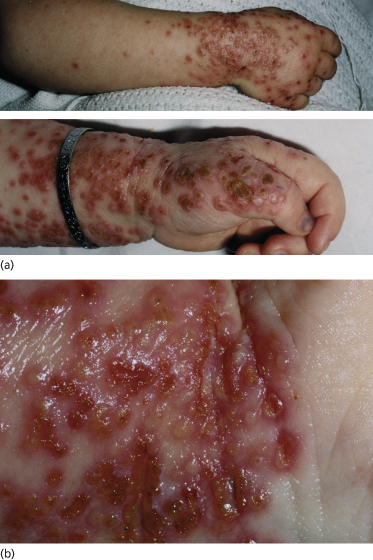
Early lesions or milder forms of smallpox could be confused with many eruptive exanthems, especially chickenpox, but a full-blown case of ordinary-type smallpox is unlikely to be missed. Children are usually extremely toxic and display the typical centrifugal distribution of lesions [3–6,9,10]. In chickenpox, the rash is centripetal, seen mainly on the trunk and rarely on the palms and soles. Unlike smallpox, there is a rapid evolution and healing of lesions in recurrent crops, so that many lesions are in different phases in the same body site. Monkeypox is almost identical to smallpox, with the same acral distribution, but with more marked adenopathy. Lesions of eczema herpeticum may all be at the same stage of development (Fig. 51.6) but are smaller and more superficial, rupturing easily and crusting within the first week. Scanning electron microscopy will rapidly differentiate between herpes- and poxviruses.
Fig. 51.6 Eczema herpeticum of the distal arm and hand. (a) The distribution mimics smallpox in this case. (b) A close-up shows the typical lesion of herpes, some of which are at the same stage of evolution, but there is already early crusting at day 4 and the lesions are smaller and less firm than smallpox (authors’ collection).
Other acral blistering disorders, such as hand-foot-and-mouth disease, are sufficiently distinctive to make confusion unlikely. In bullous erythema multiforme major, the lesions are not uniform in size or shape. The lesions on the palms and soles of secondary syphilis are of varying sizes and do not progress to form blisters. Haemorrhagic smallpox, however, could easily be mistaken for leukaemia or other purpuric conditions such as meningitis, vasculitis or even drug reactions. Breman & Henderson [10] give a clear account of the differential diagnosis and management of smallpox. In addition, public health websites such as those of the WHO [11] or the USA Centers for Disease Control and Prevention (CDC) [5] have excellent photographic images and an algorithm for the differential diagnosis of smallpox.
Investigations.
The variola virus can sometimes be detected in swabs from the nasopharynx as early as 5–6 days before the development of rash. The patient is not infectious at this time [4]. In addition, lesional swabs, blister fluid, scabs, blood and urine should be taken, using the precautions and equipment supplied by regional smallpox teams. These should be transported by special arrangements to a designated reference laboratory. Electron microscopy will quickly identify the presence of an orthopox virus, but polymerase chain reaction (PCR) will be required for rapid diagnosis of variola virus [12,13]. Isolation of virus on live cell cultures or growth on chorio-allantois is slow and outdated and nucleic acid identification is still required to distinguish between different orthopoxviruses. At present, serological testing does not differentiate between orthopox species or between recent infection and past vaccination, although in the future newer IgM detection methods may help to do so [10].
Pathology.
Multiplication of virions within the cellular cytoplasm of the basal and lower spinous keratinocytes produces the typical cytoplasmic eosinophilic inclusion bodies common to all orthopox infections [14]. In variola, they are small and located close to the nucleus, surrounded by a clear halo (Guarnieri’s bodies) [14]. Similar bodies occur in vaccinia. Severe intracellular oedema, ballooning and cell necrosis occur, producing microscopic vesicles, which enlarge and coalesce to form large unilocular vesicles. During the pustular phase, the vesicles become filled with large numbers of neutrophils. Destruction occurs mainly in the epidermis and superficial dermis, so that hair follicles and eccrine tissue can recover. However, the more superficial sebaceous glands are destroyed. In the later stages, granulation tissue is seen around the necrotic areas and causes deeply pitted scars. Electron microscopy demonstrates two forms, the encapsulated or ‘C’ form (the most infectious) and the non-encapsulated or mulberry ‘M’ form, which is more common (approximately 80%) (Fig. 51.1a).
Treatment.
Smallpox is a hazard group 4 organism and suspected cases should be treated as an international health emergency [4,5,10], with immediate isolation at the place of diagnosis and notification to locally designated smallpox teams.
Protocols are now in place in most countries for the management of suspected cases of smallpox, with designated regional teams for isolation, assessment, treatment, decontamination and vaccination of contacts. Detailed descriptions are beyond the scope of this article and readers are referred to the appropriate public health authority guidelines or website in their own country.
Hospital patients should be nursed in an environment of high-efficiency particulate air (HEPA) filtration by previously vaccinated staff [4]. Vaccination of all contacts and their contacts, a process known as ring vaccination, should be undertaken [3,4,10]. Fluid replacement and attention to skin and eye care are essential. Although its effect in smallpox is unproven, topical idoxuridine could be used for corneal lesions [3,10]. Patients should be observed for signs of septicaemia, hypotension or purpura and treated appropriately with general supportive measures. Secondary infection and pneumonia require antibiotic therapy. Vaccinia immune globulin (VIG), which was used in the treatment of smallpox and complications of vaccination in the 1970s [3], is not currently recommended during either the incubation period or clinical stages of smallpox and should be reserved for the complications of vaccination [4,5]. In the 1960s, semithiocarbazone (marboran) derivatives were used to treat smallpox, but their benefit was not substantiated [4]. The DNA polymerase inhibitor cidofovir given intravenously in the first days after exposure has been shown to be effective against most poxviruses in the laboratory [15] and in theory could be used to treat smallpox. However, this medication has significant renal toxicity and should be administered with probenecid and hydration. It is not known to be more effective than vaccination in the immediate postexposure phase [4]. Patients who die from smallpox should be cremated as soon as possible and mortuary workers vaccinated [4].
References
1 Hopkins DR. Princes and Peasants. Smallpox in History. Chicago: University of Chicago Press, 1983.
2 Henderson DA, Moss B. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA (eds) Vaccines, 3rd edn. 1999.
3 Fenner F, Henderson DA, Arita I et al. Smallpox and its Eradication. Geneva: World Health Organization, 1988. www.who.int/emc/diseases/smallpox/Smallpoxeradication.html.
4 Henderson DA, Inglesby TV, Bartlett JG et al. Smallpox as a biological weapon. JAMA 1999;281:2127–37.
5 Centers for Disease Control and Prevention. Smallpox home page. http://www.bt.cdc.gov/agent/smallpox/index.asp.
6 Dixon CW. Smallpox. London: Churchill, 1962. www.nlm.nih.gov/nichsr/esmallpox/esmallpox.html.
7 Baxby D. Jenner’s Smallpox Vaccine: the Riddle of Vaccinia Virus and its Origin. London: Heinemann Educational Books, 1981.
8 Wehrle PF, Posch J, Richter KH et al. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull World Health Organ 1970;43:669–79.
9 Rao AR. Smallpox. Bombay: Kothari Book Depot, 1972. www.nlm.nih.gov/nichsr/esmallpox/esmallpox.html.
10 Breman JG, Henderson DA. The diagnosis and management of smallpox. N Engl J Med 2002;346:1300–8.
11 WHO Slide Set on Diagnosis of Smallpox. www.who.int/emc/diseases/smallpox/slideset/.
12 Espy MJ, Cockerill FR III, Meyer RF et al. Detection of smallpox virus DNA by LightCycler PCR. J Clin Microbiol 2002;40:1985–8.
13 Ropp SL, Jin Q, Knight JC et al. PCR strategy for identification and differentiation of smallpox and other orthopoxviruses. J Clin Microbiol 1995;33:2069–76.
14 McKee PH. Pathology of the Skin with Clinical Correlations, 2nd edn. London: Mosby-Wolfe, 1996: 4–14.
15 De Clercq E. Cidovir in the therapy and short-term prophylaxis of the poxvirus infections. Trends Pharmacol Sci 2002;23:456–8.
Vaccinia and Vaccination
Historical Aspects of Vaccination.
Variolation, which originated in the East, was widely used in the second half of the 18th century as a method of introducing live smallpox material superficially into the skin. The intention was to produce a milder form of the disease. However, it was not without hazard and often led to full-blown clinical smallpox, with considerable morbidity and a mortality of between 1 in 70 and 1 in 200 [1,2]. Non-immune contacts were particularly at risk.
Edward Jenner (1749–1823) was the first to publish work on the use of cowpox material as a protective inoculation against smallpox [1,2]. This later became known as vaccination (vacca is Latin for cow). Jenner had noted that dairy maids who had previously contracted cowpox appeared to be immune to smallpox. In 1796, he used material taken from a cowpox lesion on a dairy maid’s hand to inoculate 8-year-old James Phipps, protecting him from subsequent variolation.
News of Jenner’s ‘vaccination’ spread rapidly throughout Europe and then the world. Despite initial opposition, it was quickly adopted in preference to variolation and this resulted in virtual eradication of smallpox in many countries [1,2]. The original vaccinators used either cowpox or horsepox material and supplies were shared and replenished when possible, so that many vaccines existed at this time [1–3]. Cowpox was rare even in Jenner’s time and vesicle fluid from successful vaccinees was used for arm-to-arm transfer, dried on cotton threads and ivory stylets or stored in quills [1–3]. Supplies of vaccine were later assured by growth on calf skin, a practice which resulted in particular outrage from the anti-vaccination lobby [2]. Secondary infection was common and reduced the vaccines’ effectiveness. In order to counteract this phenomenon, vaccination was often performed at multiple sites until the 1930s. The introduction of glycerated calf serum considerably reduced the risk of infection and in the 20th century mass vaccination with stable freeze-dried vaccines using vaccinia virus was both more effective and less dangerous. This replaced the older vaccines [2–4].
Vaccinia and Modern Vaccination.
Vaccinia is an orthopox virus 300–400 nm in diameter. Its origins are unknown but it possibly originated from an extinct form of horsepox [1,2]. Several different strains of a lyophilized preparation of live Vaccinia virus (VACV) are available for use [5,6]. During vaccination, VACV is inserted into the basal layers of the epidermis for maximum effect.
Many different techniques have been employed with varying effectiveness and morbidity. In the 1960s and 1970s, scarring following vaccination with rotary methods, commonly employed in India, was sometimes due to trauma rather than successful vaccination [3]. Latterly, superficial scarification or multiple punctures (usually 5–15) with a two-tined or bifurcated needle (Fig. 51.7a) were the most common procedures [3–5]. Currently, 2–3 punctures are recommended for primary vaccination and 15 for revaccination. Prior treatment of the skin with alcohol inactivates the virus and should not be used. In primary vaccination [4,7], an initial papule evolves to a vesicle and then a pustule. The erythematous areola reaches a maximal size at 8–10 days (Fig. 51.7b). Systemic symptoms, with malaise, myalgia, mild pyrexia (up to 39°C) and localized soreness and itching, occur between days 4 and 14 in over 70% of children. Regional lymphadenopathy is common and may be associated with localized lymphangitis (Fig. 51.7c). The scab separates between 14 and 21 days, leaving a puckered scar (Fig. 51.7d). Not infrequently, there are small satellite lesions around the primary site (Fig. 51.7e).
Fig. 51.7 (a) Bifurcated needle. (b) Typical primary take reaction at days 6–10. (c) Lymphangitis around vaccination site sometimes occurs and is associated with localized lymphadenopathy. (d) Smallpox vaccination scars. (e) Satellite scars around vaccination site are considered a variant of normal. (a) Courtesy of Dr Derrick Baxby. (b,c,e)
Reproduced with permission from the CDC, Atlanta, GA, USA. (d) Author’s collection.
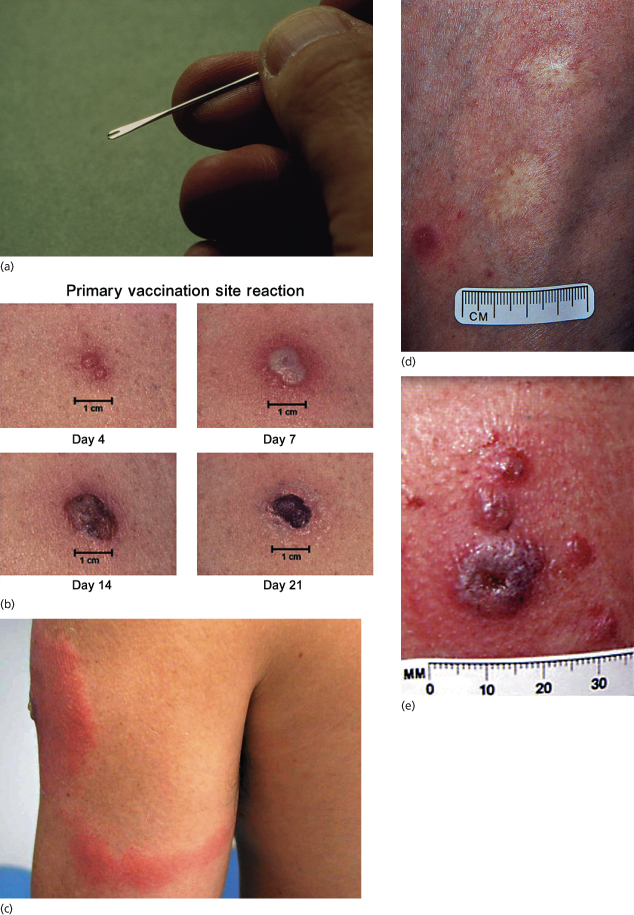
Vaccination site reactions are classified into two categories: major reactions and equivocal or ‘non-take’ reactions. Response to vaccination should be observed between days 6 and 8 and anything less than a major reaction should be considered as equivocal and the individual should be revaccinated. There are several reasons for ‘non-takes’, including suboptimal techniques, suboptimal vaccine quality or persistence of residual immunity in previously vaccinated individuals [7]. Hypersensitivity reactions with localized erythema, maximal at 48 h, indicate a failure of ‘primary take’ and these individuals should also be revaccinated [4].
In about 10% of patients, around 8–10 days after vaccination, there may be considerable oedema and severe erythema greater than 10 cm. This is indicative of a viral cellulitis, known as a robust take (RT) and is considered a normal variant rather than an adverse reaction. This must be differentiated from much rarer secondary bacterial cellulitis, which is more common in children and usually occurs within 5 days of vaccination [7].
Smallpox vaccination is successful in producing some immunity in over 95% of patients, but there is wide individual variation and protection is limited to a few years in some vaccinees [4]. Laboratory workers in contact with vaccinia should be revaccinated every 10 years [6,8], but current recommendations for health workers is every 3 years. Information on smallpox vaccination and its complications can be found on the CDC website [7].
Genetically engineered smallpox vaccine, which codes for the immunizing antigen of rabies virus, has been used to control wild fox rabies in Belgium. This attenuated vaccinia vector has limited capacity for replication and is not thought to represent a public health hazard [9]. Other recombinant vaccinia viruses are being studied but none has yet been licensed for use in humans [10].
Protective Immune Responses to Variola and Vaccinia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








