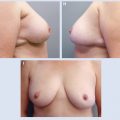
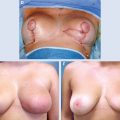
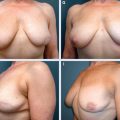
5 Breast-Conserving Therapy
Since 1894, when William Halsted 1 first described the radical mastectomy, surgical resection for breast cancer has been the mainstay of treatment. In fact, radical mastectomy was the operation of choice for all breast malignancies for the next 75 years. Fortunately, advances in breast screening, increased knowledge of tumor physiology, and better patient education have led to the earlier detection of breast cancer. As a result, many of the tumors requiring treatment are smaller and less advanced. In addition, surgical and medical oncologists in the later half of the twentieth century began to seek alternatives to standard treatments, including ever smaller resection sizes (modified radical mastectomy and simple mastectomy) and the addition of new modalities (chemotherapy and radiotherapy). Breast-conserving therapy (BCT) is a culmination of these efforts and has been validated by multiple, well-controlled, prospective randomized clinical trials as being an equally safe and efficacious treatment for early-stage breast cancer. 2 – 9
BCT involves excising a tumor with negative pathologic margins. This can be performed with or without an axillary staging procedure and is usually followed by adjuvant radiation. Successful BCT for invasive breast cancer includes the following:
Tumor resection with negative margins
Ipsilateral node assessment
Preservation of healthy parenchyma and breast aesthetics
Successful treatment is measured by local and distant disease-free survival, cosmesis, and patient satisfaction. If established guidelines for BCT are followed, survival is equal to that of mastectomy, recurrence rates should be less than 1% per year, and cosmesis and patient satisfaction should be equal to or better than conventional treatments.
Indications and Patient Selection
The use of BCT for early-stage breast cancer is widely accepted and is now the treatment of choice in the United States. 10 Most early-stage breast cancers (T1 and T2 cancers with or without nodal involvement) are amenable to conservation therapy; however, there are exceptions. Traditionally, absolute contraindications to BCT were (1) patients with a high probability of recurrence, especially those with multicentric disease, 11 and (2) patients who are pregnant, those with collagen vascular disease, or those who have a history of prior radiation. 12 Relative contraindications include (1) patients with a high probability of subsequent cancers (BRCA mutations) and (2) patients who are likely to have a poor cosmetic result, which includes patients with a high tumor/breast ratio, 13 – 16 medially and inferiorly based tumors, 17 , 18 and tumors that require removal of the nipple-areola complex. Finally, age is an independent prognostic factor that can increase the incidence of failure and the risk of metachronous breast cancer.
The addition of neoadjuvant chemotherapy and recent advances in oncoplastic surgical techniques is changing these contraindications and increasing the range of patients amenable to conservation therapy. Preoperative chemotherapy has demonstrated complete pathologic response rates of up to 30% 19 and significant tumor regression in 40% to 70% of patients. 20 In clinical trials, up to 90% of non-BCT candidates were eligible for BCT after induction chemotherapy. 21 , 22 For patients with multicentric disease, induction chemotherapy and BCT have shown survival and recurrence rates equal to those of mastectomy. 23 BCS following neoadjuvant chemotherapy has been demonstrated to be equally oncologically safe compared to mastectomy for high stage patients and broadens the indications for BCS for high stage patients. 24 , 25 The optimal timing of breast reconstruction following BCS or mastectomy for patients requiring radiotherapy is unclear; both implant and autologous reconstruction may be negatively impacted by radiotherapy and therefore are often delayed. 26
Oncoplastic techniques integrate oncologic principles with aesthetic principles. The combination of surgical oncology and plastic surgery broadens the indications for BCT and minimizes the potential for poor aesthetic results.
Examples include patients with larger tumors and tumors in cosmetically sensitive locations. Together, these modalities aid in achieving the goals and increasing the success of conservation therapy.
Technique
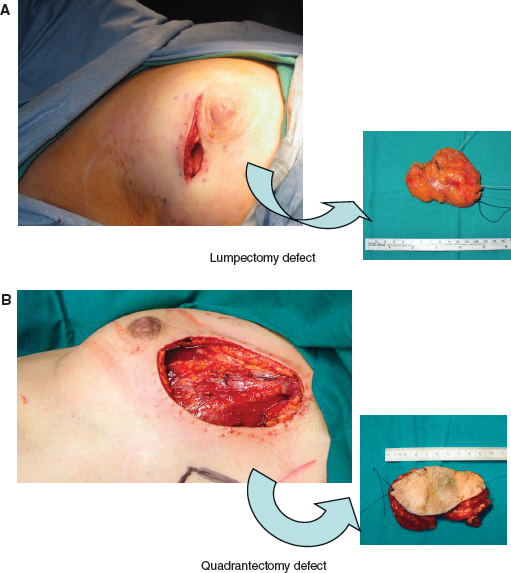
BCT entails the complete removal of the breast tumor with a concentric margin of healthy tissue, performed with more attention to aesthetics and usually followed by radiotherapy. 27 The evaluation of axillary nodes is customarily performed, and thus the current and most widely practiced technique entails wide local excision (also called lumpectomy, segmentectomy, or partial mastectomy), sentinel lymph node biopsy, and postoperative radiotherapy totaling 45 to 50 Gy with a boost to the tumor bed (A). Quadrantectomy refers to removal of an additional 1 to 2 cm of healthy parenchyma around the tumor (B). This technique is more frequently performed in Europe and has been demonstrated to give a lower rate of local recurrence; however, cosmetic results are worse with quadrantectomy when compared with lumpectomy.
Tumor Resection
Excision of the tumor with negative margins is essential, and it is accepted that additional procedures may be necessary to achieve this goal. A recent consensus conference stated that most oncologists were comfortable with a 1 to 2 mm margin. 27 However, the width of the negative margin remains controversial and the term close margin has been coined for cancers within 1 mm of the resection edge.
A concentric or transverse skin incision should be made directly over the tumor or in close proximity in an attempt to “hide” the scar (periareolar incision for central or medial tumors and axillary incision for tail tumors). Tumors can be marked with clips or wires from a previous biopsy or mammogram-guided placement to identify tumor location. Dissection should proceed directly to the cancer without tunneling, and resection should include a concentric portion of healthy parenchyma. Once removed, the specimen is oriented and marked for pathologic review; we prefer to use a long marking stitch on the lateral aspect of the specimen and a short stitch superiorly. Hemostasis is obtained, and the margins of resection are marked with radioopaque clips. The wound is packed and covered, and attention is then turned to the axilla while the specimen is sent for frozen section analysis. If the resection margin is clear of cancer, the superficial skin and fascia are closed, and then the remaining defect is left to fill with serous fluid.
The Axilla
The axilla is considered separately. Axillary node analysis helps in staging, treating, and dictating adjuvant therapies.
The identification of positive axillary nodes indicates a poorer prognosis and often leads to additional adjuvant treatments.
With the patient in the supine position, the boundaries of the axilla include the latissimus dorsi muscle posteriorly, the lateral edge of the pectoralis muscles anteriorly, the serratus muscle medially, the axillary vein cranially, and the tail of the breast caudally. Important structures within the axilla include the axillary artery and vein; the long thoracic nerve, which innervates the serratus anterior muscle; the thoracodorsal nerve, which innervates the latissimus muscle; and the intercostobrachial nerve, which innervates the lower arm. The axillary lymph nodes are categorized according to their relation to the pectoralis minor muscle: level I lateral, level II inferior, and level III medial.
The sentinel node is the first node to receive lymphatic drainage from the tumor site and can be identified by color or radioactive emission. The first technique requires injecting 3 ml of blue dye (isosulfan blue or methylene blue) into the tumor site, followed by gentle massage for several minutes to increase lymphatic drainage. After creating the axillary incision and identifying the pectoralis major muscle, the surgeon performs a delicate search for any blue-stained nodes. Stained nodes—often there are several—are removed and sent to pathology. For the second technique, approximately 5 ml of technetium-labeled sulfur colloid is injected into the tumor bed 2 to 4 hours before the operation. A handheld gamma detection probe is used to identify the “background noise” and the area of highest activity in the axilla.
The axillary incision is made over the site of high activity, keeping in mind that a complete axillary dissection may be necessary.
Dissection is guided by the detection counter, and the “hottest” node is identified and removed. Additional nodes measuring greater than 10% of the sentinel node are removed, and the resection continues until the nodal basin emits less than twice the background. The finding of a positive node dictates a complete level I and level II axillary dissection. A consensus conference has stated that the standard for sentinel node biopsy ought to include both technetium and blue dye, which, when combined, is more than 95% accurate. 28
Axillary dissection is achieved through a small transverse incision that is centered in the axilla and spans the borders of the pectoralis and latissimus muscles. Superior and inferior skin flaps are developed through sharp dissection, and retractors are placed to open the wound. Identifying and dissecting free the lateral border of the pectoralis major muscle allows its retraction and aids in exposing the adjacent lymph nodes (Rotter nodes) and the underlying pectoralis minor muscle. Retracting the pectoralis minor muscle after ligating the medial pectoral nerve allows access to the level II nodes. The level I nodes are isolated by meticulous dissection of the axillary vein from lateral to medial. Continuing the dissection inferiorly allows identification first of the thoracodorsal nerve (running with the subscapular vein) and then of the long thoracic nerve. Once the axillary contents are removed and oriented, hemostasis is obtained, a drain is placed within the cavity, and the skin and superficial fascia are closed.
Radiotherapy
Postoperative radiotherapy is an integral part of the multidisciplinary approach to cancer treatment.
The goal of radiation is to decrease the local recurrence risk to below 1% per year.
A standard course delivers approximately 50 Gy to the whole breast over a 5-week period, followed by 2 weeks of an additional boost of approximately 15 Gy to the tumor bed. A recent advancement, called partial breast irradiation, delivers the same amount of radiation in 5 days through intracavitary catheters, balloon catheters, or an external beam. Although it is currently being used, partial breast irradiation has not yet undergone a long-term evaluation. Radiation is given either in continuous low doses or fractionated high doses.
Outcomes
Because BCT and mastectomy are equivalent local treatments for most patients, the ultimate choice is not only a medical choice but also a quality-of-life decision made by the patient. The success of conservation therapy is measured by patient survival, the local failure rate, cosmesis, and the patient’s satisfaction.
*p = 0.01.
†p <0.001.
‡The recurrence rates for lumpectomy alone and lumpectomy plus XRT were 39.2% and 14.3%, respectively. AXD, Axillary dissection; BCT, breast-conserving therapy; Quad, quadrantectomy; XRT, radiotherapy.
Many prospective, randomized trials have proven that BCT provides a survival benefit similar to mastectomy for early-stage breast cancer. 2 – 9 The survival of breast cancer patients depends on prognostic factors such as nodal metastases, the tumor size, the tumor grade, and the systemic therapy. 12 , 29
Local recurrence rates after BCT are higher than after a mastectomy. The goal set forth by the Consensus Conference on Breast Conservation is a 10-year local recurrence rate between 5% and 10%, 27 which translates into a risk of developing a recurrence of 1% per annum—a good percentage to use when counseling patients.
Local recurrence in BCT is higher, especially when postoperative radiotherapy is omitted (39.2% versus 14.3%) 4 ; however, local failures can be salvaged with a mastectomy at no detriment to survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree