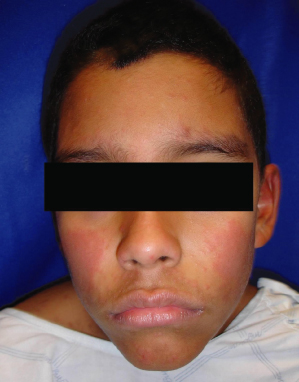
The characteristic eruption of measles consists of red macules and papules starting on the face and neck, especially behind the ears and along the hairline. It then spreads caudally to the rest of the body, often becoming confluent on the face and trunk (Fig. 49.2). Lesions gradually fade in the same order after 4–5 days, leaving behind coppery macules and a fine desquamation. The fever usually resolves 2–3 days after onset of the rash, but cough can persist for 10 days or longer [2,9]. Other possible symptoms include photosensitivity, anorexia and generalized lymphadenopathy.
Fig. 49.2 The measles exanthem at day 3, showing confluence on the face and upper trunk.
Reproduced with permission from the Centers for Disease Control and Prevention.
Secondary complications are seen in up to 40% of cases [10]. Morbidity and mortality are directly related to the nutritional status of the patient and age of disease acquisition. Children less than 5 years of age and adults 20 years of age and older are particularly affected. The most frequent complications are diarrhoea, otitis media and bronchitis. Death occurs in 0.3% of patients, with pneumonia accounting for the most cases. Acute encephalitis can affect 0.1% of persons within 2 weeks of the rash and give fever, seizures, headache and even coma. A higher incidence is seen in adolescents and adults, causing death in 15–25% and lifelong neurological sequelae such as motor impairment and mental retardation in 33% of survivors [10,11]. While conjunctivitis and keratitis are common and generally self-limited, corneal ulceration and blindness may manifest in individuals in developing countries [12], where vitamin A deficiency is prevalent, and appears to predispose to more severe infection.
Months to years after the acute disease, there can be a rare complication called subacute sclerosing panencephalitis from persistent measles virus infection. It most often occurs in those infected under the age of 2 years and slowly progresses from seizures to deterioration of cognitive and motor functions, followed by death. Individuals with defective cellular immunity may develop measles inclusion body encephalitis 5 weeks to 6 months after acute measles. Mental status changes and seizures develop without fever and over 80% of deaths occur within 1 week [13,14].
In addition to typical measles, mild/modified and atypical forms also occur. Modified measles can occur in young infants with residual maternal antibodies, in those receiving immunoglobulin therapy and in adults with partial immunity from prior vaccination. The incubation period is longer, the prodrome milder and the eruption sparse. Atypical measles occurred in children from the 1960s to the 1980s when formalin-inactivated (killed) measles vaccines were in use. They developed high fever, a rash most prominent on the extremities that often included petechiae and a high rate of pneumonitis. This was thought to be from antigen-antibody immune complexes resulting from incomplete maturation of the antibody response to the vaccines [9,15]. It is now rare with the substitution of live, attenuated vaccines for the previously utilized killed form.
Differential Diagnosis.
Koplik’s spots are considered pathognomonic for measles, though their presence has also been documented with erythema infectiosum [16]. The latter, however, has a distinct eruption characterized by a generalized, reticulated erythematous rash and confluent erythema of the face (‘slapped cheeks’). Rubella has a less striking rash and shorter course, fading as it spreads. Rocky Mountain spotted fever should also be considered in the differential for atypical measles, but the rash typically spares the face. Other infections, such as Epstein–Barr virus and Mycoplasma infections, may mimic measles as well, but without the complete set of features. Drug eruptions often have a morbilliform appearance, but in uncomplicated cases lack cough or other prodromal symptoms. Kawasaki’s disease has fever, irritability, conjunctivitis and a morbilliform eruption, but also peripheral oedema of the extremities and palmoplantar erythema; Koplik’s spots do not occur.
Laboratory Findings.
Histological findings from skin include a non-specific superficial perivascular lymphocytic infiltrate, with variable spongiosis and dyskeratosis. Finding multinucleated keratinocytes is helpful for diagnosis of measles. Giant cells, called Warthin–Finkelday cells, may be seen in the tonsils, lymph nodes and other reticuloendothelial tissues [17].
Serological testing for immunoglobulin M (IgM) antibody and examination of throat/nasopharyngeal cells for viral antigens by immunofluorescent microscopy may aid diagnosis. Polymerase chain reaction (PCR) may be used to detect the measles virus in nasopharyngeal and urine samples; viral culture, however, is difficult and much less sensitive [7].
Treatment and Prevention.
Symptomatic care with attention to hydration and nutrition is required. Natural disease confers lifelong immunity in immunocompetent individuals. To prevent spread, otherwise healthy children with measles should be isolated until 4 days after the onset of the eruption, whereas immunocompromised individuals need to be isolated for the duration of the illness.
Active immunization is advocated to prevent measles. Current recommendations are two doses of live, attenuated virus vaccine given after 12 months of age; waning immunity is rare with this schedule. Because of the high transmissibility of measles virus, immunization levels of the order of 95% are necessary to prevent outbreaks within a particular population [18,19]. Some parents have declined vaccination of their children because of concerns relating to autism, but numerous studies do not support either the measles-mumps-rubella vaccine or thimerosal, a vaccine preservative, as a primary cause of this condition [20–22].
In suspected or confirmed exposures, the measles vaccine may provide some protection if given within 72 hours. Susceptible household contacts and persons at high risk of complications (e.g. immunosuppressed individuals, infants <1 year of age, pregnant women) should be given immune globulin (IVIG) within 6 days. Two doses of oral vitamin A given 24 hours apart may benefit children in areas where vitamin A deficiency is a recognized problem or where the measles case fatality rate is 1% or higher [23,24].
References
1 Bialecki C, Feder HM Jr, Grant-Kels JM. The six classic childhood exanthems: a review and update. J Am Acad Dermatol 1989;21:891–903.
2 Cunha BA. Smallpox and measles: historical aspects and clinical differentiation. Infect Dis Clin North Am 2004;18:79–100.
3 De Quadros CA, Izurieta H, Carrasco P, Brana M, Tambini G. Progress toward measles eradication in the region of the Americas. J Infect Dis 2003;187(suppl 1):S102–10.
4 World Health Organization. Measles. www.who.int/immunization_monitoring/diseases/measles/en/index.html. Accessed March 24, 2009.
5 Centers for Disease Control and Prevention (CDC). Outbreak of measles – San Diego, California, January – February 2008. MMWR 2008;57:203–6.
6 Atkinson WL. Epidemiology and prevention of measles. Dermatol Clin 1995;13:553–9.
7 Rota PA, Featherstone DA, Bellini WJ. Molecular epidemiology of measles virus. Curr Top Microbiol Immunol 2009;330:129–50.
8 Steichen O, Dautheville S. Koplik spots in early measles. CMAJ 2009;180:583.
9 Arguedas AG, Deveikis AA, Marks MI. Measles. Am J Infect Control 1991;19:290–8.
10 Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004;189(suppl 1):S4–16.
11 Miller DL. Frequency of complications of measles. BMJ 1963;2:75–8.
12 Foster A, Sommer A. Corneal ulceration, measles and childhood blindness in Tanzania. Br J Ophthalmol 1987;71:331–43.
13 Rima BK, Duprex WP. Morbilliviruses and human disease. J Pathol 2006;208:199–214.
14 Modlin JF, Jabbour JT, Witte JJ, Halsey NA. Epidemiologic studies of measles, measles vaccine and subacute sclerosing panencephalitis. Pediatrics 1977;59:505–12.
15 Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 2003;9:1209–13.
16 Evans LM, Grossman ME, Gregory N. Koplik spots and a purpuric eruption associated with parvovirus B19 infection. J Am Acad Dermatol 1992;27:466–7.
17 Lightwood R, Nolan R. Epithelial giant cells in measles as an aid in diagnosis. J Pediatr 1970;77:59–64.
18 Centers for Disease Control and Prevention (CDC). Update: Measles – United States, January–July 2008. MMWR 2008;57:893–6.
19 Anderson RM, May RM. Infectious Diseases of humans. Dynamics and Control. Oxford: Oxford University Press, 1992: 70.
20 Farrington CP, Miller E, Taylor B. MMR and autism: further evidence against a causal association. Vaccine 2001;19:3632–5.
21 Demicheli V, Jefferson T, Rivetti A, Price D. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev 2005;19:CD004407.
22 Harappanahally GV, Trask CL, Mandelbaum DE. Vaccines and autism: an update. Med Health R I 2007;90:308–10.
23 Duke T, Mgone CS. Measles: not just another viral exanthem. Lancet 2003;361:763–73.
24 Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005;19:CD001479.
Rubella
Rubella, also known as German measles, was first recognized as a separate human disease in 1814. The causative virus is an RNA virus in the Togaviridae family and unlike the measles virus, rubella virus is only moderately contagious. It is also spread by the respiratory route and causes disease in humans only; there is no animal or insect reservoir. Up to 50% of infections are subclinical [1].
Epidemiology and Pathogenesis.
Incidence of disease is usually highest in late winter and early spring. After entry into the upper respiratory tract, viral replication occurs in the nasopharyngeal mucosa and regional lymph nodes. Viraemia then occurs 5–7 days after exposure followed by spread to the skin and other organs of the body, including the placenta in pregnant women. An individual is most contagious when the rash first appears, but shedding of virus can continue for weeks after the rash resolves [2].
Clinical Features.
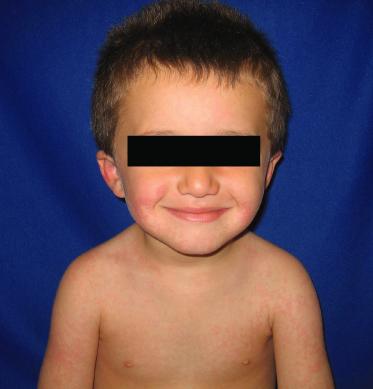
The incubation period of rubella is 12–23 days. In young children, there may not be a prodrome, but adults tend to experience low-grade fever and malaise. A rash appears soon after, with pink macules and sometimes papules starting on the face and spreading caudally. Lesions do not coalesce and are fainter than the lesions of measles (Fig. 49.3). They tend to fade after 1–3 days. Lymphadenopathy is commonly seen, especially the postauricular and occipital nodes.
Fig. 49.3 The rash of rubella is less red than that of measles.
Reproduced with permission from the Centers for Disease Control and Prevention.
Complications of postnatal infection include arthralgias/arthritis and encephalitis, but are much more common in adults than in children [3]. Arthalgias tend to occur at about the same time or soon after the rash. The joints of the hands, wrists and knees are often affected and symptoms may last up to 1 month or longer, but chronic arthritis is rare [4].
The major concern with rubella is not childhood infection, but congenital disease. Complications range from spontaneous abortion to premature birth and deformity and are determined by the point in gestation at which infection occurs. Risk of congenital rubella is as high as 65% if infection occurs in the first 16 weeks of pregnancy [3,5]. It is one of the TORCH infections causing blueberry muffin lesions (extramedullary haematopoiesis) and also a major cause of congenital cataracts. Low birthweight, chorioretinitis, sensorineural deafness, cardiac defects (patent ductus arteriosus and septal defects) and pulmonary stenosis may be seen as well. Some manifestations, such as deafness, may progress and others, such as developmental abnormalities and diabetes mellitus, may not be detected until the second year of life or later [6].
Differential Diagnosis.
Infection with parvovirus B19 is difficult to distinguish clinically from rubella, since fever, rash and joint symptoms commonly occur in both conditions. However, confluent erythema of both cheeks is more likely with parvovirus. Pregnant women with a history of exposure or developing non-vesicular rashes should be investigated for both infections. Rubella tends to have a rash of shorter duration than measles. In exanthema subitum, the rash appears with resolution of fever, rather than at the peak. Infectious mononucleosis can be distinguished by atypical lymphocytosis on laboratory testing. Most drug eruptions are pruritic and lack the prominent lymph node enlargement seen with rubella.
Laboratory Findings.
A transient thrombocytopenia may occur during rubella infection. Given the clinical uncertainty that usually exists and the potential risk to pregnant women, it is important to confirm the diagnosis by laboratory studies. The only reliable evidence of acute rubella infection in children is detection of virus by polymerase chain reaction, rubella-specific IgM antibody or a significant rise in immunoglobulin G (IgG) antibody from paired acute and convalescent sera. Virus may be isolated from the pharyx from 1 week before to 2 weeks after rash onset. Serology is the most common method of confirming diagnosis, but false-positive IgM has occurred with parvovirus infection, with positive heterophile testing in infectious mononucleosis and with a positive rheumatoid factor [3,7]. Viral cultures are more labour intensive and thus are not generally used for routine diagnosis. Congenital rubella infection is confirmed by the presence of IgM in the newborn as, unlike IgG, IgM does not cross the placenta from the mother [8].
Treatment and Prevention.
Symptoms of postnatal rubella are usually mild without necessitating treatment. Children should be excluded from school for 7 days after the onset of rash. Congenital cases may need multidisciplinary management and because they are likely to excrete high concentrations of virus, these newborns should be nursed in isolation to avoid spread [1,9].
Prevention is best accomplished by vaccination and long-term vaccine efficacy is more than 90% [10]. The current recommended immunization schedule is two doses of the live vaccine given in conjunction with the measles and mumps vaccines. Incidence of congenital rubella has markedly declined with vaccination, but if a pregnant woman has been exposed to rubella, serological testing should be performed. If rubella-specific IgM antibodies or a diagnostic rise in anti-rubella IgG antibodies is detected, the patient should be offered prenatal counselling. Human normal immunoglobulin or rubella hyperimmune globulin (if available) soon after exposure may reduce the amount of viraemia and damage, but the incidence of fetal infection does not seem to be reduced [11,12].
References
1 Best JM. Rubella. Semin Fetal Neonatal Med 2007;12:182–92.
2 Centers for Disease Control and Prevention. Rubella. In: Atkinson W, Hamborsky J, McIntyre L, Wolfe S (eds.) Epidemiology and Prevention of Vaccine-Preventable Diseases, 10th edn. Washington, DC: Public Health Foundation, 2008.
3 Banatvala JE, Brown DW. Rubella. Lancet 2004;363:1127–37.
4 Tingle AJ, Allen M, Petty RE, Kettyls GD, Chantler JK. Rubella-associated arthritis: comparative study of joint manifestations associated with natural rubella infection and RA27/3 immunisation. Ann Rheum Dis 1986;45:110–14.
5 Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982;2:781–4.
6 Duszak RS. Congenital rubella syndrome – major review. Optometry 2009;80:36–43.
7 Dwyer DE, Robertson PW, Field PR, Board of Education of the Royal College of Pathologists of Australasia. Broadsheet: clinical and laboratory features of rubella. Pathology 2001;33:322–8.
8 Tang J, Aarons E, Hesketh LM et al. Prenatal diagnosis of congenital rubella infection in the second trimester of pregnancy. Prenat Diagn 2003;23:509–12.
9 Cooper LZ, Alford CA. Rubella. In: Remington JS, Klein JO, Wilson CB, Baker CJ (eds) Infectious Diseases of the Fetus and Newborn Infant, 6th edn. Philadelphia: Elsevier, Saunders, 2006: 894–926.
10 O’Shea S, Woodward S, Best JM et al. Rubella vaccination: persistence of antibodies for 10–21 years. Lancet 1988;2:909.
11 Peckham CS. Clinical and serological assessment of children exposed in utero to confirmed maternal rubella. BMJ 1974; 2:259–61.
12 Centers for Disease Control and Prevention. Rubella prevention. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR 1990;39:1–18.
Erythema Infectiosum
The distinct clinical features of erythema infectiosum, or fifth disease, were recognized in the 19th century. The causative agent was not determined until much later, when human parvovirus B19 was discovered in 1975 while screening blood donors for hepatitis B antigen and disease linkage was established 8 years later [1,2]. Parvovirus B19 is a single-stranded DNA virus in the Parvoviridae family and has tropism for cells with a high mitotic rate, such as erythroid progenitor cells. It is very resistant to heat and detergent inactivation. Twenty-five to 50% of infections are asymptomatic [3].
Epidemiology and Pathogenesis.
Infection occurs most commonly among school-aged children (4–10 years old), with a seasonal peak during late winter to early spring. Transmission is predominantly via respiratory droplets, but at times is also from contact with contaminated hands or infected blood products or bone marrow [4,5]. Virus is shed in nasopharyngeal secretions during times of viraemia, which occur 4–5 days after infection with peak levels 2–3 days later. With erythema infectiosum in an immunocompetent host, specific antibody production and clearance of viraemia coincide with appearance of the rash and the affected individual is no longer contagious. In contrast, immunocompromised individuals and those with a specific subset of parvovirus infection, papular-purpuric gloves and socks syndrome (PPGSS), continue to be infectious for a more prolonged period (see below) [3].
Clinical Features.
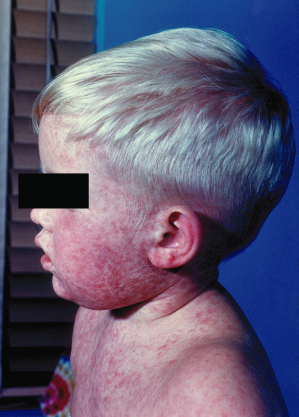
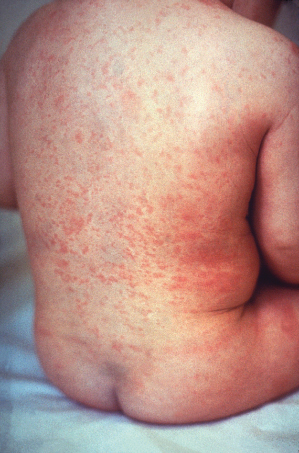
The prodromal symptoms of erythema infectiosum are mild and include fever, coryza, headache and nausea. An enanthem with erythema of the tongue and pharynx and red macules on the buccal mucosa and palate (Koplik’s spots) may occur [6]. The typical rash proceeds through three stages: the first stage presents as erythema of the cheeks with circumoral pallor (‘slapped cheeks’, Fig. 49.4). After 1–4 days, pink macules and papules appear on the extensor sides of the extremities and on the trunk, often with central clearing of the rash to give a lacy/reticular pattern (Fig. 49.5). This second stage usually lasts from 1 to 6 weeks. The third stage is persistence of the skin lesions, ranging from 1 to 3 weeks. The eruption then resolves spontaneously with no permanent sequelae. Adults often have a less pronounced rash than affected children. Occasionally the rash has been reported to recur for several months following exposure to heat, sunlight, exercise and other triggers [4,7].
Arthritis can be an associated symptom of erythema infectiosum, but can also occur as the only manifestation of B19 infection in adults [8]. Other systemic findings include hepatitis and lymphadenopathy.
Other Parvovirus B19 Issues.
Though self-limited in healthy individuals, infection with parvovirus B19 can give severe or poor outcomes in three groups of patients: the fetus, those with haemoglobinopathies and the immunocompromised. Fetal infection can lead to hydrops, profound anaemia, heart failure and ultimately death. Fetal loss has been reported with primary maternal infection in all three trimesters of pregnancy, but the highest risk appears to be during the first 20 weeks of gestation [9]. Fortunately, most maternal infections are associated with normal pregnancy outcomes, with overall risk of adverse outcome estimated at 4–9% in studies [9,10].
Because of its preference for erythroblasts, B19 infection can cause a transient aplastic crisis in those with sickle cell anaemia and other conditions of accelerated haematopoiesis and shortened red cell survival (hereditary spherocytosis, thalassaemias, etc.). In immunocompromised patients, the infection can become chronic because of an inability to mount an adequate neutralizing antibody response. The virus persists in the serum or bone marrow; chronic anaemia or red cell aplasia can occur, along with leucopenia and thrombocytopenia [11,12]. Because of viral persistence, these individuals remain contagious and able to transmit the virus to others.
Differential Diagnosis.
As discussed earlier, rubella infection needs to be distinguished from erythema infectiosum, but the former tends to progress and fade over 2–3 days. Measles has Koplik’s spots and does not give a reticular eruption. Scarlet fever tends to have a more sandpaper-like rash with accentuation in body folds. Erysipelas of the cheeks can appear similar to the slapped cheeks of erythema infectiosum, but is typically unilateral and hot to touch and does not progress to a generalized eruption. Drug reactions may sometimes be difficult to distinguish from parovivus B19 infection, as are the collagen vascular diseases, and appropriate testing is needed to determine cause.
Laboratory Findings.
Laboratory diagnosis of acute/recent infection is by detection of anti-B19 IgM. Seroconversion from IgG negative to positive is also indicative of recent infection. Because the virus requires a mitotically active host cell for replication, it cannot be cultured in standard cell lines [13]. Polymerase chain reaction can be used to detect B19 DNA in immunocompromised individuals who are unable to mount an adequate humoral response. PCR techniques may also be useful for maternal and fetal serum analysis when serological study results are unclear. B19 antigen detection in amniotic fluid samples has also been described [10,14].
Treatment and Prevention.
Immunocompetent children with erythema infectiosum do not generally require treatment beyond analgesics and anti-inflammatory medications for joint and other systemic symptoms. Blood transfusions may be needed in cases complicated by aplastic anemia. IVIG is helpful for immunosuppressed individuals who lack neutralizing antibodies, as it contains pooled antibody against the virus [15,16]. Pregnant women who have been exposed to affected individuals during the infectious phase should have serum antibody titres performed. If IgG is present (over 50% of the adult population are immune), the family can be reassured regarding the mother’s immune status. If she is non-immune or seropositive for IgM, weekly ultrasound studies to assess for fetal anaemia and hydrops should be performed. If anaemia is seen, intrauterine erythrocyte transfusions can be performed via the umbilical vein [10,17].
Prevention includes routine hygiene practices. Pregnant women are not routinely excluded from the workplace because transmission occurs before onset of the rash of erythema infectiosum. Droplet precautions to prevent transmission are recommended for those caring for children with aplastic crisis or immunosuppression. Candidate vaccines have been studied, but are not yet fully developed for use [18].
References
1 Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet 1975;1:72–3.
2 Anderson MJ, Lewis E, Kidd IM, Hall SM, Cohen BJ. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984;93:85–93.
3 Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. J Intern Med 2006;260:285–304.
4 Katta R. Parvovirus B19: a review. Dermatol Clin 2002;20:333–42.
5 Heegaard ED, Petersen Laub B. Parvovirus B19 transmitted by bone marrow. Br J Haematol 2000;111:659–61.
6 Evans LM, Grossman ME, Gregory N. Koplik spots and a purpuric eruption associated with parvovirus B19 infection. J Am Acad Dermatol 1992;27:466–7.
7 Koch WC. Fifth (human parvovirus) and sixth (herpesvirus 6) diseases. Curr Opin Infect Dis 2001;14:343–56.
8 Kerr JR. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis 2000;59:672–83.
9 Miller E, Fairley CK, Cohen BJ et al. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol 1988;105:174–8.
10 Ergaz Z, Ornoy A. Parvovirus B19 in pregnancy. Reprod Toxicol 2006;21:421–35.
11 Florea AV, Ionescu DN, Melhem MF. Parvovirus B19 infection in the immunocompromised host. Arch Pathol Lab Med 2007;131:799–804.
12 De Renzo A, Azzi A, Zakrzewska K et al. Cytopenia caused by parvovirus in an adult ALL patient. Haematologica 1994;79:259–61.
13 Peterlana D, Puccetti A, Corrocher R, Lunardi C. Serologic and molecular detection of human Parvovirus B19 infection. Clin Chim Acta 2006;372:14–23.
14 Hsu ST, Chen YT, Huang YF et al. Prenatal diagnosis and perinatal management of maternal-fetal congenital parvovirus B19 infection. Taiwan J Obstet Gynecol 2007;46:417–22.
15 Kurtzman G, Frickhofen N, Kimball J et al. Pure red-cell aplasia of 10 years’ duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. N Engl J Med 1989;321:519–23.
16 Koduri PR, Kumapley R,Valladares J, Teter C. Chronic pure red cell aplasia caused by Parvovirus B19 in AIDS: use of intravenous immunoglobulin – a report of eight patients. Am J Hematol 1999;61:16–20.
17 Enders M, Weidner A, Zoellner I, Searle K, Enders G. Fetal morbidity and mortality after acute human Parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn 2004;24:513–18.
18 Ballou WR, Reed JL, Noble W, Young NS, Koenig S. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C. J Infect Dis 2003;187:675–8.
19 Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–97.
Roseola Infantum
Roseola infantum is also called exanthema subitum or sixth disease. Most cases are caused by human herpesvirus-6 (HHV-6), a virus discovered in 1986 and found to cause roseola 2 years later (usually type 6B) [1]. The other cases are associated with human herpesvirus-7 infection; both are double-stranded DNA viruses in the β-herpesvirus family that have tropism for T lymphocytes. HHV-6 and 7 remain in the host body for life after primary infection and may be reactivated, particularly with immunosuppression [2].
Epidemiology and Pathogenesis.
Human herpesvirus-6 and 7 are spread by contaminated saliva and less commonly by blood or stem cell transplantation. HHV-6 primarily infects children from the time of waning of maternal antibodies at 6 months of age to 24 months of age, while HHV-7 infection occurs later, in the first 5–6 years of life [3]. This correlates well with the tendency of roseola to affect those between 6 months and 3 years of age. No seasonal variation is noted with this condition.
Clinical Features.
After a 5–15-day incubation period, the child develops sudden high fever (to 40–40.5°C) that lasts for 3–5 days. Upper respiratory symptoms, irritability, diarrhoea and cervical lymphadenoapathy may occasionally be present. Nagayama’s spots are erythematous papules on the mucosa of the soft palate and uvula that occurs in up to two-thirds of patients [4]. A characteristic feature of roseola is the abrupt appearance of a cutaneous eruption coincident with subsiding of the fever. The rash consists of rose-pink macules and papules that blanch on pressure and are surrounded by a white halo. The neck and trunk are particularly affected; the rash evolves over about 12 hours and begins to fade within a day or two. Oedema can be seen at the palpebral and periorbital areas (Berliner’s sign) and may be a useful clue to diagnosis.
With roseola, there is an approximately 10% risk of febrile seizures [5]. It is unclear if the seizures are secondary to fever or to the infection itself. Rarely, exanthema subitum can be associted with hepatitis, pneumonitis, neuropathy and encephalopathy. Though most with central nervous system involvement have a normal recovery, permanent sequelae such as hemiparesis have been reported to occur [5,6].
Differential Diagnosis.
Echovirus, particularly the 16 subtype, can give a roseola-like rash but has a higher frequency of upper respiratory symptoms, conjuctivitis, vomiting and aseptic meningitis [7]. The differential diagnosis includes other viral infections such as adenovirus, rubella, rubeola or parainfluenza, but the temporal characteristics of roseola are distinctive.
Laboratory Findings.
Because appearance of the rash is coincident with defervescence, there is usually little need to pursue further studies. A leucopenia may be present, with a lower number of both neutrophils and lymphocytes. The cerebrospinal fluid is usually normal in children with associated febrile seizures [8]. Routine serological tests for HHV-6 and 7 infection have difficulty in determining reactivation versus primary infection, as rises in IgM and IgG titres can occur in either case. IgG antibody avidity tests may be needed, with avidity low in recent primary infection but high if primary infection occurred at least 6 weeks previously. Antigens from the two viruses may cross-react and make it difficult to differentiate between them [3].
Treatment and Prevention.
Fevers should be controlled and anticonvulsants used in the case of seizures. Antiviral agents are not recommended except in cases of serious disease in immunocompromised and transplant patients, with some reports of response to ganciclovir and foscarnet therapy [9,10].
References
1 Yamanishi K, Okuno T, Shiraki K et al. Identification of human herpesvirus-6 as a casual agent for exanthem subitum. Lancet 1988;1065–7.
2 Caselli E, di Luca D. Molecular biology and clinical associations of Roseoloviruses human herpesvirus 6 and human herpesvirus 7. New Microbiol 2007;30:173–87.
3 Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and 7 infections in the immunocompetent. J Clin Virol 2005;32:183–93.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree