Clinical Features and Prognosis.
Eosinophilic pustular folliculitis (Fig. 36.2) is characterized by recurrent crops of intensely pruritic annular or polycyclic plaques, composed of coalescing, sterile papulopustules on the seborrhoeic areas of the face, trunk and extremities. There is a tendency for central healing with peripheral extension. Scalp involvement is present in only 6% of adult patients [37]. This classic form has been reported in older children and adolescents [4,29,31,33,37–45] rarely.
Fig. 36.2 Eosinophilic pustular folliculitis: (a) on the scalp; (b) face; (c) trunk; and (d) limbs. Note the sparing of the skin creases.
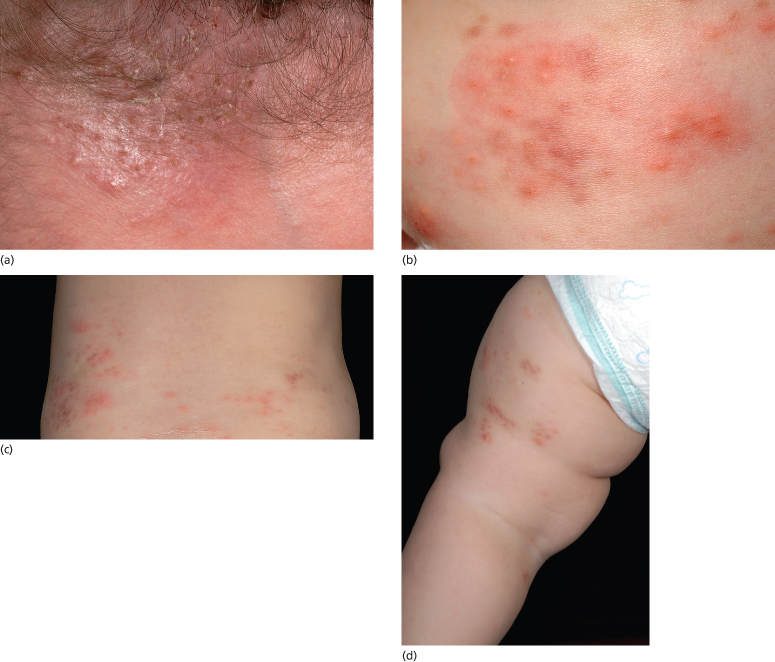
Eosinophilic pustular folliculitis in infancy appears to be a variant of this disorder, which principally involves the scalp [3–15,32,35]. These lesions, similar to those in the adult form, occur as grouped, pruritic firm papules (occasionally vesicles), which evolve into follicular pustules, measuring pinpoint to 3 mm, on an erythematous base. The lesions can present as a solitary plaque or as scattered pustules throughout the scalp [20]. Unlike the adult and childhood forms, annular or serpiginous plaques are not observed in infants and small children. They resolve spontaneously without scarring after evolving through a yellow, crusted phase in approximately 5–10 days, often leaving a 3–4 mm hyperpigmented macule. New crops of lesions appear approximately every 2–8 weeks. In addition to the scalp, the majority of infants have involvement of the extremities, hands, feet and trunk. The face [4,16,33], groin [6] and genitals [4] may be affected. Mucosal involvement was noted in one 7-year-old child [40]. In the infantile variant, onset is most likely in the first 6 months of life, with approximately one-quarter presenting at or just after birth [10,11]. Spontaneous resolution occurs mostly between 4 and 36 months. It appears that the earlier the disorder appears, the less chronic it becomes. Although there does not seem to be a familial predisposition, eosinophilic pustular folliculitis has been reported in two brothers, born at different times, both with onset in the neonatal period [32].
In all age groups there is peripheral eosinophilia and leucocytosis associated with exacerbations and an absence of systemic symptoms. Both classic and infantile forms exhibit a strong male predominance; however, in the 4–9 year age group, which represents by far the smallest cohort, girls have been reported more often. The clinical characteristics of eosinophilic pustular folliculitis in this age group fall between the other groups in that scalp involvement is unusual, seborrhoeic areas are less involved than in the classic form and there are fewer annular and more diffuse lesions as seen in the infantile form [37].
A Wright’s-stained smear of pustular contents demonstrating abundant eosinophils may be helpful in making the diagnosis. The diagnosis is based on the clinical presentation, associated haematological abnormalities and histological evidence of eosinophilic folliculitis.
The term necrotizing eosinophilic folliculitis has recently been coined for a variant of eosinophilic folliculitis presenting with ulcerating nodules. Magro and Crowson [46] described 10 patients, one of whom was an 11-year-old girl, who had in common a history of atopy and evidence of follicular and dermal necrosis with an eosinophilic vasculitis on biopsy of their ulcerated nodules.
Differential Diagnosis.
The differential diagnosis for eosinophilic pustular folliculitis in infancy should include most pustular eruptions in infancy, in particular scalp pyoderma or infectious folliculitis caused by Staphylococcus aureus, scabies, erythema toxicum neonatorum, transient neonatal pustular melanosis, herpes simplex infection, infantile acropustulosis, Langerhans cell histiocytosis, incontinentia pigmenti, hypereosinophilic syndrome and drug eruptions.
When eosinophilic pustular folliculitis presents in older children, the differential diagnosis should include fungal, parasitic and herpetic infections, eosinophilic cellulitis, insect bites, eczema, impetigo, lymphoma and drug eruptions.
Treatment.
Because of the rarity of this disorder, there have been no controlled clinical trials for the treatment of eosinophilic pustular folliculitis in adults or children. In addition, its recurrent and self-limited features, especially in infants, make evaluation of anecdotal treatments difficult. Antihistamines are indicated for associated pruritus and there may be a role for the eosinophil antimigration drugs such as cetirizine dihydrochloride [6,47]. The H2-antagonist cimetidine was used with success in a 9-month-old girl [48]. Although not consistently effective, most have found that mid- to high-potency topical steroids reduce pruritus and hasten involution of the lesions in infants [3,16,33], with response usually within 1–2 days of application. Topical calcineurin inhibitors have been successfully used in adolescents and adults [49]. Dapsone, at a dose of 2 mg/kg per day, was effective in one infant who was unresponsive to topical steroids; however, it was discontinued secondary to haematological side-effects. The eruption recurred after the dapsone was stopped [32]. Responses to oral antibiotics (penicillin G, erythromycin, cephalexin) have been variable [3,15,16,33].
In adults, it has been suggested that non-steroidal anti-inflammatory agents such as indometacin and naproxen are the agents of choice [16,50]. In patients with HIV-associated disease, there is generally a good response to antiretroviral therapy but eosinophilic pustular folliculitis can occur with immune reconstitution. Other treatment options include topical and systemic steroids, isotretinoin, diphenylsulphone, sulphamethoxazole, oxyphenbutazone, minocycline, doxycycline, metronidazole, itraconazole, cyproheptadine, colchicine, glycyrrhizin, interferon-α2b, interferon-γ and ciclosporin. Topical permethrim 5% cream, transdermal nicotine patches and, for the acquired immunodeficiency syndrome (AIDS)-associated variety, phototherapy (PUVA (psoralens and ultraviolet A) and narrow band) may be successful [33,51]. Radiation was helpful in a recalcitrant case [52].
References
1 Ise S, Ofuji S. Subcorneal pustular dermatosis. A follicular variant? Arch Dermatol 1965;92:169–71.
2 Ofuji S, Ogino A, Horio T et al. Eosinophilic pustular folliculitis. Acta Dermatol Venereol 1970;50:195–203.
3 Lucky A, Esterly NB, Heskel N et al. Eosinophilic pustular folliculitis in infancy. Pediatr Dermatol 1984;1:202–6.
4 Taieb A, Bassan-Andrieu L, Maleville J. Eosinophilic pustulosis of the scalp in childhood. J Am Acad Dermatol 1992;27:55–60.
5 Taieb A. Infantile eosinophilic pustular ‘folliculitis’ in infancy: a nonfollicular disease (Letter). Pediatr Dermatol 1994;11:186.
6 Larralde M, Morales S, Santo Munoz A et al. Eosinophilic pustules folliculitis in infancy: report of two new cases. Pediatr Dermatol 1999;16:118–20.
7 Korshid SM, Glover M, Cerio R. Infantile eosinophilic pustular folliculitis: report of the first British case. Eur J Dermatol 1997;7:385–7.
8 Vicente A, Espana E, Idoate M et al. Are eosinophilic pustular folliculitis of infancy and infantile acropustulosis the same entity? Br J Dermatol 1996;135:807–9.
9 Coulson IH, Ling TC, Stringfellow HF. Case 2: infantile eosinophilic pustular folliculitis (IEPF). Clin Exp Dermatol 2002;27(1):80–1.
10 Picone Z, Madile BM. Eosinophilic pustular folliculitis (Ofuji’s disease) in a newborn. Pediatr Dermatol 1992;9:178.
11 Buckley DA, Munn SE, Higgins EM. Neonatal eosinophilic pustular folliculitis. Clin Exp Dermatol 2001;26:251–5.
12 Ziemer M, Boer A. Eosinophilic pustular folliculitis in infancy: not a distinctive inflammatory disease of the skin. Am J Dermatopathol 2005;27:443–55.
13 Luelmo AJ, Saez AA. Eosinophilic pustular folliculitis in childhood. An Esp Paediatr 2001;55:154–8.
14 Rybojad M, Guibal F, Vignon-Pennamen MD et al. Eosinophilic pustulosis in an infant accompanied by immune deficit Ann Derm Venereol 1999;126:29–31.
15 Garcia-Patos V, Pujol RM, de Moragas JM. Infantile eosinophilic pustular folliculitis. Dermatology 1994;189:133–8.
16 Duarte AM, Kramer J, Yusk JW et al. Eosinophilic pustular folliculitis in infancy and childhood. Am J Dis Child 1993;147:197–200.
17 Fearfield LA, Rowe A, Francis N et al. Itchy folliculitis and human immunodeficiency virus infection: clinicopathological and immunological features, pathogenesis and treatment. Br J Dermatol 1999;141:3–11.
18 Bull RH, Harland CA, Fallowfield M et al. Eosinophilic folliculitis: a self limiting illness in patients being treated for haematological malignancy. Br J Dermatol 1993;129:178–82.
19 Otley CC, Avram MR, Johnson RA. Isotretinoin treatment of human immunodeficiency virus associated eosinophilic folliculitis. Arch Dermatol 1995;2131:1047–50.
20 Ramdial PK, Morar N, Dlova NC et al. HIV-associated eosinophilic folliculitis in an infant. Am J Dermatopathol 1999;21:241–6.
21 Kishimoto S, Yamamoto M, Nomiayama T et al. Eosinophilic pustular folliculitis in association with nevoid basal cell carcinoma syndrome. Acta Dermatol Venereol 2001;81:202–4.
22 Takematsu H, Tagami H. Eosinophilic pustular folliculitis: studies on possible chemotactic factors involved in the formation of pustules. Br J Dermatol 1986;114:209–15.
23 Miyauchi T, Fujigaki H, Uehara N et al. Effect of indomethacin on eosinophilic folliculitis. Acta Dermatol (Kyoto) 1985;80:9–13.
24 Ota T, Hata Y, Tanikawa A et al. Eosinophilic pustular folliculitis (Ofuji’s disease): indomethacin as a first choice treatment. Clin Exp Dermatol 2001;26:179–81.
25 Amerio P, Verdolini R, Proietto G et al. Role of Th2 cytokines, RANTES and eotaxin in AIDS-associated eosinophilic folliculitis. Acta Derm Venereol 2001;81:92–5.
26 Amerio P, Frezzolini A, Feliciani C et al. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutic implications. Curr Drug Targets Inflamm Allergy 2003;2:81–94.
27 Vakilzadeh F, Suter L, Knop J et al. Eosinophilic pustulosis with pemphigus-like antibody. Dermatologica 1981;162:265–72.
28 Nunzi E, Parodi A, Rebora A. Ofuji’s disease: high circulating titers of IgG and IgM directed to the basal cell cytoplasm. J Am Acad Dermatol 1985;12:268–73.
29 Boone M, Dangoisse C, Andre J et al. Eosinophilic pustular folliculitis in three atopic children with hypersensitivity to Dermatophagoides pteronyssinus. Dermatology 1995;190:164–8.
30 Maruo K, Kayashima KI, Ono T. Expression of neuronal nitric oxide synthase in dermatol infiltrated eosinophils in eosinophilic pustular folliculitis J Dermatol 1999;140:417–20.
31 Ishiguro N, Shishido E, Okamoto R et al. Ofuji’s disease: a report of 20 patients with clinical and histopathologic analysis. J Am Acad Dermatol 2002;46:827–33.
32 Dupond AS, Aubin F, Bourezane Y et al. Eosinophilic pustular folliculitis in infancy: report of two affected brothers. Br J Dermatol 1995;132:296–9.
33 Giard F, Marcoux D, McCuaig C et al. Eosinophilic pustular folliculitis (Ofuji disease) in childhood: a review of four cases. Pediatr Dermatol 1991;8:189–93.
34 Onorato J, Heilman ER, Laude TA. Pruritic pustular eruption in an infant (clinical conference). Pediatr Dermatol 1994;10:292–4.
35 Daremstadt GL, Tunnessen WW, Sweren RJ. Eosinophilic pustular folliculitis. Pediatrics 1992;89:1095–8.
36 Lee JY, Tsai YM, Sheu HM. Ofuji’s disease with follicular mucinosis and its differential diagnosis from alopecia mucinosa. J Cutan Pathol 2003;30:307–13.
37 Dekio S, Jidoi J, Kawasaki Y. Eosinophil-infiltrating folliculitis in childhood: report of a case. J Dermatol 1989;16:388–91.
38 Takematsu H, Nakamura K, Igarashi M et al. Eosinophilic pustular folliculitis: report of two cases with a review of the Japanese literature. Arch Dermatol 1985;121:917–20.
39 Hsu PJ, Huang CJ, Wu MT. Pathergy in atypical eosinophilic pustular folliculitis. Int J Dermatol 2005;44:203–5.
40 Boudaya S, Turki H, Bouassida S, Khemakhem M, Marrakchi S, Zahaf A. Eosinophilic pustular folliculitis in infancy: an unusual case. Ann Dermatol Venereol 2003;130:451–4.
41 Boone M, Dangoisse C, Andre J, Sass U, Song M, Ledoux M. Ofuji’s disease and eosinophilic pustular folliculitis. Ann Dermatol Venereol 1992;119:780–5.
42 Lazarov A, Wolach B, Cordoba M, Abraham D, Vardy D. Eosinophilic pustular folliculitis (Ofuji’s disease) in a child. Cutis 1996;58:135–8.
43 Jang KA, Chung ST, Choi JH, Sung KJ, Moon KC, Koh JK. Eosinophilic pustular folliculitis (Ofuji’s disease) in myelodysplastic syndrome. J Dermatol 1998;25:742–6.
44 Souissi A, Fenniche S, Benmously R et al. Eosinophilic pustular folliculitis in a child. Tunis Med 2008;86:190–2.
45 De Dulanto F, Armijo M, Diaz L et al. Eosinophilic pustular folliculitis. Med Cutan Ibero Lat Am 1977;5:323–30.
46 Magro CM, Crowson AN. Necrotizing eosinophilic folliculitis as a manifestation of the atopic diathesis. Int J Dermatol 2000;39:672–7.
47 Fadel R, Herpin-Richard N, Rihoux JP et al. Effect of cetirizine on eosinophil migration in vivo. Clin Allergy 1987;17:373–9.
48 Rogers M. Successful treatment of eosinophilic pustulosis with oral cimetidine. Paediatr Dermatol 1999;16:335–6.
49 Kawaguchi M, Mitsuhashi Y, Kondo,S. Successful treatment of eosinophilic pustular folliculitis with topical tacrolimus. Int J Dermatol 2004;43:608–10.
50 Youn CS, Cho KH. Eosinophilic pustular folliculitis treated with naproxen. Br J Dermatol 2001;145:514–15.
51 Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol 2004;5:189–97.
52 Wilson BD, Kucera JC, Shin PJ. The role of radiation treatment in the management of eosinophilic pustular folliculitis. J Med 2002;33:111–13.
Idiopathic Hypereosinophilic Syndrome
Definition.
Recent advances in cytogenetics, immunology and molecular biology have led to the classification of primary eosinophilia as clonal, reactive and idiopathic [1]. Secondary hypereosinophilia is common in allergic disorders, parasitosis and in association with bullous pemphigoid, Churg–Strauss syndrome, scabies and drug eruptions.
The majority of genetically defined eosinophilic diseases with recurrent molecular abnormalities result in constitutively activated fusion tyrosine kinases, the phenotypic consequence of which is an eosinophilia-associated myeloid disorder. Most notable among these is the recent discovery of the cryptic FIP1L1-PDGFRA gene fusion in karyotypically normal patients, defining these diseases as clonal eosinophilias. Rearrangements involving PDGFRA and PDGFRB in eosinophilic chronic myeloproliferative disorders, and of fibroblast growth factor receptor 1 (FGFR1) in the 8p11 stem cell myeloproliferative syndrome, constitute additional examples of specific genetic alterations linked to clonal eosinophilia [2,3].
The identification of populations of aberrant T-lymphocytes secreting eosinophilopoietic cytokines such as interleukin-5 establishes a pathophysiological basis for cases of lymphocyte-mediated hypereosinophilia. Such reactive eosinophilia is associated with lymphoma (including cutaneous T-cell lymphoma (CTCL)) or abnormal, often clonal T lymphoid populations. Idiopathic eosinophilia occurs in the hypereosinophilic syndrome (HES) which is a diagnosis of exclusion with unexplained hyperesosinophilia associated with tissue damage.
Hypereosinophilic syndrome is a multisystem disease with a high mortality rate. Chusid and co-workers [4] formulated a definition with strict criteria for the diagnosis of HES as follows.
1 Peripheral blood eosinophilia more than 1500 cells/cu mm for at least 6 months’ duration.
2 Symptoms and signs of end-organ involvement with eosinophil tissue infiltration/injury of the skin and principally the heart (40–70%) [5–7] and lungs [4]. The muscles [8], gastrointestinal tract [9–12], eyes, nasopharynx [13], bone marrow and central nervous system [14–17] may be involved. Rarely, the kidneys [18] and bladder [19] are involved.
3 Exclusion of known secondary causes of eosinophilia.
Aetiology and Pathogenesis.
It is still not clear whether eosinophils or their precursors themselves are abnormal in HES, or whether there is a problem in the regulatory mechanism of eosinophil production. The striking male preponderance (9:1) also remains unexplained. The mean age at onset is 37 years and the disorder is distinctly rare in childhood [20–23]. It is an important syndrome to recognize as eosinophilic infiltration of the cardiac tissue induces fibrosis and resultant restrictive cardiomyopathy. In the series reported by Alfaham, 16 of the 18 children had cardiac involvement and, untreated, this carries a high mortality [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








