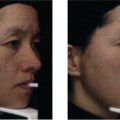
Carl R. Thornfeldt Episciences, Inc., Boise, ID, USA Herbs are the 14,000 species of higher plants with extracts for medicinal, fragrance, or flavoring use [1]. The growing interest in herbal products has resulted in significant growth of the number of commercially available natural products in our domestic market to over 90,000. These products are based on over 1100 commercially available herbs. Six wholesale companies each market over 100 herbal extracts, with one company selling over 1000 [2]. Despite the increase in the number of herbal products, the usage rate appears to be flattening after rapid growth over the past 6 years. Supplement consumption rose from 33.8% in the general population in 1990, to 55% in those seeking cosmetic procedures in 2006. The incidence of supplement use in the latter population declined to 49% in 2012. Unfortunately, 60–72% of supplement users do not report these to their physician preoperatively for cosmetic procedures [3, 4]. Herb‐based products provide the advantage of having multiple functionalities in stable blends of highly reactive ingredients such as antioxidants and antimicrobials. While it is difficult to create efficacious and stable cosmeceutical formulations, herbs contain blends of multiple, often unique, active ingredients that are biologically active [2, 4]. Since most skin conditions and diseases are multifactorial with multiple mechanisms of action inducing the visible changes, herbal products would be expected to produce visible benefit. While 73% of women polled said it is important to have younger looking skin, the vast majority of topical botanical products promise youth but lack true clinical evidence. This evidence is defined as blinded clinical trials of statistically significant numbers, using the finished, marketed product. Studies with individual ingredients in a laboratory vehicle system are not valid due to the chemical complexity of topical formulations that impact ingredient chemistry and function [5]. A surprising 78.7% of women polled did not know claims made for skin care products are not regulated, evaluated, or verified by any governmental agency. Only in the USA are herbal products sold as dietary supplements which are governed by the Dietary Supplement, Health and Education Act in 1994. Such products are intended to affect the structure and function of the human body, as well as provide calories and nutrients. Topically applied herbs for treating cosmetic skin conditions are commonly referred to as cosmeceuticals. These products improve the appearance of skin like a cosmetic, but modulate surface skin morphology like a drug. Yet there is no official regulatory category for cosmeceutical ingredients. Certain ingredients used in cosmeceuticals may be claimed to be therapeutic for common skin diseases. These ingredients are regulated as over the counter (OTC) drug monographs for acne, dermatitis/psoriasis, skin protectant, topical analgesia and sunscreens, by the Food and Drug Administration (FDA). Non‐prescription products that list these active ingredients that are used in the specified concentration range in topical formulations may claim the finished product to be effective and safe therapy for the specific skin conditions listed. The United States has a regulatory classification for food supplement and cosmetic ingredients with proven safety, called Generally Recognized as Safe, or GRAS [5]. CFR Title 21 – Food Drugs and Cosmetic Act regulates skin care products and cosmetics, including cosmeceuticals. These products are not supposed to harm the public. Cosmeceuticals are actually helpful to the skin. According to this regulation, mislabeling a cosmetic is illegal. Mislabeling is defined as labeling the cosmetic with false or misleading information, or the label does not include all the information required by this Act. There are also four other statutes that do not apply to topicals. The FDA has become more aggressive in regulating topicals, thus experts are calling for revision and updating CFR Title 21. Products that claim to affect gene expression and DNA are among those under scrutiny as drugs. Recently, warning letters addressing cosmetics that make drug claims have been issued. Additionally, the FDA is monitoring social media, which resulted in a citation for using Facebook to promote unapproved “new” drugs, defined as “making therapeutic claims.” Moreover, such regulatory monitoring is needed as more herbal products with actives which are known to have toxic components are being marketed. These apparently are without any basic safety data presented in any communication medium. Cosmetics can only claim to temporarily improve appearance [6]. There is no consistent definition of “natural” in US skin care products, so it is marketing jargon without functionality [7]. Recently a recommended criterion is, “5% or more of the ingredients are found in nature.” Organic products are ones that are certified free of synthetic chemical use in the fields, and using production methods of the ingredients obtained in nature [1]. The US Department of Agriculture now has certification criteria for this designation to include certified as 100% organic, but can claim organic if 95% of components are. Organic‐derived can be claimed if 70% of the components are organic [8]. There has been no decision by the FDA about genetically modified foods labeled as “natural.” The skin care practitioner should select a herb for a specific desired beneficial effect based on scientific research and/or traditional medical knowledge founded on ethnobotany. As interest in phytomedicines for improving health as well as treating disease is growing rapidly, many “exotic” species with certain “fad” characteristics are being touted also for cosmeceuticals. Fortunately, most of these exotic ingredients are added at subtherapeutic doses just for the marketing story of the product. Little is known about efficacy and safety of many of these ingredients. The active ingredients of plants used for human skincare are generally small molecules not involved in regular metabolic processes, known as secondary metabolites (SM). These ingredients are used for nutrient storage or organism protection. The goal of extraction processes is to improve consistency of SM concentration, increase SM potency, and increase product purity [1]. Herbal extracts are much more susceptible to quality variations than synthetic products due to environmental and processing factors affecting solubility, stability, pharmacokinetics, pharmacology, and toxicity of the active ingredients. These include: Additional details are provided in the previous edition of this book [9]. The processing of herbal extracts provides a variety of products, including: Patients and clients entrust skincare professionals to create effective and safe regimens for their skin, yet there are no safety requirements for cosmeceuticals. Best corporate practices should ensure safety studies are performed on all herbal products. The incidence of dangerous adverse reactions to herbal medicines is infrequent, but is growing. The most common adverse reaction consists of 40% of US women who claim to have sensitive skin [1]. German chamomile (Matricaria recutita), cayenne (Capsicum annuum), and echinacea (Echinacea angustifolia) have been reported to produce anaphylactic reactions even to death. Taborandi (Pilocarpus microphyllus) induced death via cardiac arrest, while poison ivy (Rhus toxicodendron) induced death via coma. Fatalities have also been reported with Aloe, Aristocholia, Arnica, Black Mustard, Cascara, Cayenne, Chinese Rhubarb, Comfrey, Croton, Kava, Ma Huang, Mistletoe, Oleander, Senna, Scotch pine, Spruce, and Yohimbe [2, 8, 10]. A product labeled as “natural” does not equal a safe product. Chinese practitioners are concerned about the well‐known side effects of hepatotoxicity, contact dermatitis, and teratogenicity which occur in up to a third of people using topical Chinese herbal preparations. Moreover, a significant number of congenital anomalies occur when these herbs are used topically during pregnancy [11]. Another risk of herbal products is the relatively high incidence of cross‐reactivity with other herbs. For example, in 106 dermatitis afflicted people, 12 were allergic to tea tree oil and all 12 had one or more patch test reactions to 10 other herbs, most commonly, lavender [12]. A preferred safety test for a cosmeceutical product would be the Repeat Insult Patch Test (RIPT) on 50 panelists to evaluate risk of contact irritant and allergic dermatitis with topical use. This is a 1‐month test that measures for irritant reactions in the first 10 days and allergic sensitization in the last 10 days. It will not predict systemic reactions such as gynecomastia due to widespread topically applied lavender in atopic children and teens used for prolonged periods [8, 13]. In the last decade a new development has been the use of blinded clinical studies to document efficacy of cosmeceutical products presented at peer‐reviewed meetings or in scientific journals. This is the most convincing scientific evidence of therapeutic efficacy. A total of 16 companies have produced 50 blinded clinical trials of herbs to treat photoaging. One company who produced the first blinded trial in 2002 has accounted for 16 of these blinded trials. Twelve of the 50 trials have compared the test herbal or herbal/synthetic blend formulations to prescription products. The most prolific company accounted for seven of these trials. Cosmeceutical efficacy claims for a herbal product being sold in your practice would ideally be supported by a double‐blind clinical trial [1, 5]. The true measure of a prescription or nonprescription drug’s efficacy has been determined by U S regulatory agencies to be a p value of <0.05. p Value is a measure of probability, also known as statistical significance. A p value of <0.05 means if you run a study 100 times, the comparable positive result will occur in at least 95% of the attempts. A highly statistically significant result is when p value is <0.001. This means running the study 1000 times produces the comparable positive result in 99.9% of the attempts [8]. A major challenge to the clinical efficacy of any herbal extract is the delivery of therapeutic concentration(s) of the desired active ingredients across the stratum corneum intact in their functional state to affect organelles, cells, receptors, metabolic and cell signaling pathways. The different solubilities, polarities, size, and architecture of the several to hundreds of multiple SM in herbal extracts creates difficulty. Moreover, the degree of biodegradability, biocompatibility, toxicity, release profile and antigenicity also vary amongst the multitude SM in one extract. Contributing to the challenge is the different SM bind to cells and receptors in different cutaneous strata. To meet this challenge of negotiating the tortuous proteohydrolipid stratum corneum, several novel methodologies have been developed that is beyond the scope of this chapter, but thoroughly analyzed in this listed reference [8]. Plants are equipped with protective defensive mechanisms, storage, color, and aroma via low molecular weight compounds called SM. These are not used in primary metabolic processes such as photosynthesis, respiration, assimilation of nutrients, transport, growth or differentiation. They usually provide protection against ultraviolet light, herbivores, parasites, pathogenic microbes, and animals. Certain fatty acids and certain carbohydrates function within both metabolic processes and as SM. About 20% of the higher plants have been studied with spectrometry, nuclear magnetic resonance, and/or X‐ray diffraction which have identified about 40,000 SM [1]. SM are divided into more than 15,500 terpenoids, 12,000 alkaloids, and 6000 phenolics, which have been chemically characterized from herbal extracts, as have 10 other chemical classes representing the other 6500; more extensive discussion is given in the previous edition of this book [1, 9]. To help separate true scientifically based safe and effective herbal‐based cosmeceuticals from “snake oil” products using voodoo science, and before making a decision on retailing or recommending any particular brand of skin care products, the practitioner should ask the following questions: The three theories of skin aging include: Reactive oxygen species, or free radicals, are reversed by antioxidants. Humans utilize 15 antioxidants, elements, and molecules. At no body site is there only high concentration of a single one; rather there exists a cycle phenomenon to pass off some of the oxidant energy among these multiple antioxidant molecules and elements [14]. To protect mitochondria and prevent their dysfunction, adequate amounts of photoprotective herbal anthocyanins such as carotenoids, and polyphenols and catechins from green tea, grape seed, resveratrol, and silymarin have documented benefit. Camptotheca acuminate (Happy tree) has been shown to downregulate telomerase [2]. About 200 herbs have anti‐inflammatory (AI) functionality with 140 of these having antioxidant activity. The other 60 include AI natural steroids, salicylates, and other non‐antioxidants. Only six herbal extracts for treating photoaging have been a sole active ingredient in tested formulations, including: coffeeberry, date palm kernel, oat, soy milk, total soy, and soy protease inhibitors. These and the other 33 herbs tested in blinded studies to treat photoaging are listed in Table 36.1. Table 36.1 Antiaging herbs with clinical studies in humans.
CHAPTER 36
Botanicals
Introduction
Regulatory
Factors affecting concentration and quality of active ingredients
Safety
Effectiveness
Mechanism of action
Cosmeceutical products
Specific herbs to treat or prevent photoaging
Aloe (Aloe barbadensis)
Apple (Malus domestica)
Avocado (Persea americana)
Black cohosh (Cimicifuga racemosa)
Blackberry (Rubis ursinus)
Blueberry (Vaccinium myrtillus): oral only
Cat’s claw (Uncaria guianensis, U. tomentosa)
Coffeeberry (Coffea arabica)a
Comfrey, or comphrey (Symphytum officinale)
Date palm kernel (Phoenix dactylifera)a
Dill (Anethum graveolens)
Flax (Linum usitatissimum)
German chamomile (Matricaria recutita) + oral
Goji (Lycium barbarum)
Grapefruit (Citrus paradisi): oral only
Grape seed (Vitis vinifera): oral only
Green tea (Camellia sinensis)a + oral
Lavender (Lavandula augustifolia)
Licorice (Glycyrrhiza glabra, G. inflate, G. uralensis): oral only and Glabridin topically
Mangosteen (Garcinia mangostana)
Meadowfoam (Limnanthes alba)
Mountain rose (Rosa canina)
Mushroom/wheat complex
Oak quercetin
Olive (Olea europaea)
Plum (Prunus domestica)
Pomegranate (Punica granatum) + oral
Raspberry (Rubus idaeus)
Safflower (Carthamus tinctorius)
Sakura leaf (Prunus speciosa)
Southern wood (Artemesia ambrosia)
Soy milk, total soy (Glycine soja, G. max), soy protease inhibitorsa + oral
Spring rest‐harrow (Ononis spinosa)
Sweet orange (Citrus sinensis): oral only
Tamarind (Tamarindus indica)
Tomato (Lycopersicon esculentum): oral only
White sandalwood (Santalum album)
White tea (Camellia sinensis) + oral
White willow (Salix alba)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree