This distinction may be artificial. Under certain circumstances, other proteins (such as food allergens) can become aerosolized (e.g. fried fish) or contaminate dust (e.g. latex), behaving like aeroallergens for children, while certain pollens, for example, cross-react with food allergens (causing oropharyngeal reactions when certain foods are eaten – the oral allergy syndrome). Exposure to a diverse spectrum of work-related dusts, gases, fumes and vapours can lead to occupational lung disease, and in addition to allergic reactions, cutaneous airborne reactions can include irritant, photoallergic and phototoxic responses. However, these are assumed to be less relevant within a paediatric setting.
Early observers noted that family members with atopy need not share a common disease pattern, supporting the suggestion that the development of these diseases may be dependent on a genetic predisposition, influenced by environmental factors. Classically, eczema is often the first manifestation of the atopic march, presenting before 1 year of age in 60% of affected individuals [3]. Several longitudinal studies provide evidence for the atopic march from eczema to the development of asthma and allergic rhinitis, with allergic sensitization being a major determinant of prognosis [4]. The steady increase in prevalence of eczema [5] has been paralleled by the increase seen in asthma, suggesting shared predisposing and/or triggering factors [6]. There is accumulating evidence that the development of one disease may be directly influenced by the pre-existence of the other and vice versa.
Recent major advances on molecular and cellular levels have not only provided insights into the role of both epidermal barrier function [7] and cutaneous immunological interactions [8] in the pathogenesis of eczema, but have also heralded a shift of focus, with skin as a major organ of allergic sensitizations and of allergic manifestations. The current model for atopic eczema incorporates genetic and environmental factors in the development of the disease. While genetic factors are less malleable, the environmental component lends itself to analysis and modification. Many non-allergic factors (such as infections, ambient temperature and humidity, emotional stress and damage to epidermal barrier function) will contribute to eczema disease severity and flares among atopic and non-atopic sufferers [9]. However, there is considerable evidence that aeroallergens (and food allergens) are pertinent eliciting factors in atopic eczema.
References
1 Johansson SG, Bieber T, Dahl R et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Clin Immunol 2004;113:832–6.
2 Flohr C, Weiland SK, Weinmayr G et al. The role of atopic sensitization in flexural eczema: findings from the international study of asthma and allergies in childhood phase two. J Allergy Clin Immunol 2008;121:141–7.
3 Kay J, Gawkrodger DJ, Mortimer MJ et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 1994;30:35–9.
4 Kusel MMH, Holt PG, de Klerk N et al. Support for 2 variants of eczema. J Allergy Clin Immunol 2005;116:1067–72.
5 Schultz-Larsen F, Hanifin J. Epidemiology of atopic dermatitis. Immunol Allergy Clin North Am 2002;22:1–24.
6 Eichenfield LF, Hanifin JM, Beck LA et al. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics 2003;111: 608–16.
7 Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol 2009;129: 543–52.
8 Ogg G. Role of T cells in the pathogenesis of atopic dermatitis. Clin Exp Allergy 2009;39:310–16.
9 Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol 2006;118:209–13.
Historical Context
Observations that aeroallergen exposure can provoke eczema were documented as early as 1918 [1]. The specific observation that inhalation of allergens could provoke an exacerbation in eczema was first made in the early 1930s [2,3]. The association between delayed hypersensitivity responses to aeroallergens and eczema was suggested by Rajka before even the early descriptions of T-cell subsets [4]. Three studies in the 1980s demonstrated for the first time the phenomenon of contact sensitivity to aeroallergens [5–7]. In all these studies, the authors established (in small and selected patient cohorts) that contact sensitivity can be demonstrated against a variety of patient/environment-specific aeroallergens and all found a correlation with a history of environmental precipitating factors.
References
1 Walker IC. Causation of eczema urticaria and angioneurotic edema by proteins other than those derived from food. Study XVIII. JAMA 1918;70:897–905.
2 Figley KG, Parkhurst HJ. Silk sensitivity with special reference to its role in atopic eczema. J Allergy 1933;5:60–72.
3 Sulzberger MB, Vaughan WT. Experiments in silk hypersensitivity and the inhalation of allergen in atopic dermatitis (neurodermatitis disseminatus). J Allergy 1934;5:554–67.
4 Rajka G. Delayed dermal and epicutaneous reactivity in topic dermatitis (prurigo Besnier) I. Delayed reactivity to bacterial and mold allergens. Acta Dermatovenereol (Stockh) 1967;47:158–62.
5 Mitchell EB, Crow J, Chapman MD et al. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet 1982;8264:127–30.
6 Reitamo S, Visa K, Kahonen K et al. Eczematous reactions in atopic patients caused by epicutaneous testing with inhalant allergens. Br J Dermatol 1986;114:303–9.
7 Adinoff AD, Tellez P, Clark RAF. Atopic dermatitis and aeroallergen contact sensitivity. J Allergy Clin Immunol 1988;81:736–42.
Epidemiological Evidence of Association between Aeroallergy and Atopic Dermatitis
The proportion of children with eczema and positive IgE allergen test (skinprick test (SPT) or radio-allergosorbent test (RAST)) has been found to vary widely in published studies with both geographical variation and disease severity [1]. Hospital-based eczema patient populations in general demonstrate higher risk of allergic sensitization than those in community-based studies [1]. Despite this wide variation in prevalence of aeroallergen sensitization, the relevant aeroallergens themselves are not significantly varied [2]. A characteristic pattern for aeroallergen sensitization is frequently observed (housedust mite > cat > grass pollen > Alternaria mould) [3].
References
1 Flohr C, Johansson SGO, Wahlgren CF et al. How “atopic” is atopic dermatitis? J Allergy Clin Immunol 2004;114:150–8.
2 Roberts G, Peckitt C, Northstone K et al. Relationship between aeroallergen and food allergen sensitization in childhood. Clin Exp Allergy 2005;35:933–40.
3 De Benedictis FM, Mranceshini F, Hill D et al. The allergic sensitisation in infants with atopic eczema from different countries. Allergy 2009;64:295–303.
Timing of Sensitization
In general, sensitization evolves in the order of exposure: food, indoor allergens, outdoor allergens. Sensitization to milk and egg most frequently occurs during the first year of life, while sensitization to aeroallergens occurs later in childhood, with increasing prevalence with age [1]. Sensitization to indoor airborne allergens (HDM and pets) often occurs at an earlier age than sensitization to pollens (tree and grass).
Beyond the age of 3 years, food allergy is frequently outgrown but sensitization to aeroallergens increases, such that although the prognosis of food allergy (particularly to egg and milk) is in general good, children with atopic eczema and sensitization to aeroallergens are likely to progress to rhinoconjunctivitis and/or asthma. Furthermore, the number and type of allergic sensitizations serve as negative prognostic factors predicting a more chronic and recalcitrant course of atopic eczema [2].
References
1 Illi S, von Mutius E, Lau S et al. The pattern of atopic sensitisation is associated with the development of asthma in childhood. J Allergy Clin Immunol 2001;108:709–14.
2 Akdis CA, Akdis M, Bieber T et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL consensus report. J Allergy Clin Immunol 2006;118:152–69.
Pathophysiology of Allergic Sensitization
One current disease model for the pathogenesis of atopic eczema postulates that allergen uptake by IgE receptor-bearing dendritic cells, followed by skin homing of cutaneous lymphocyte antigen (CLA)-bearing T-cells, plays a central role in initiating the inflammatory process [1]. Dendritic cells found in atopic eczema lesional skin express the high-affinity receptor for IgE (FcεRI) [2].
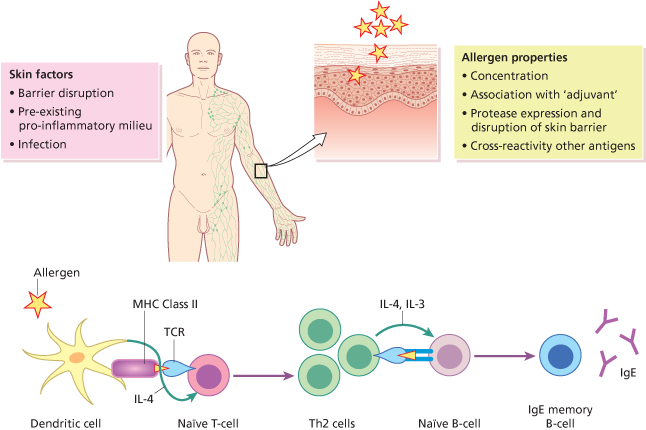
Allergy is thought to be triggered initially by encounter with an allergen. The first contact with this antigen leads to the formation of antigen-specific T-cells, predominantly Th2, and the consecutive induction of IgE-producing B-cells (Fig. 32.1). A second encounter with the allergen results in an inflammatory reaction which in turn leads to the clinical manifestations of the disease (Fig. 32.2) [3,4]. Dendritic cells are essential for priming and Th2 differentiation of naïve T-cells towards aeroallergens. Contamination of antigens with pattern-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) as well as locally produced damage-associated molecular patterns (DAMPs), such as uric acid and adenosine triphosphate (ATP), will augment an immune response (see Fig. 32.1). Several studies have recently demonstrated an important role of endogenous danger signals at the inception and maintenance phase of allergic disease. These factors may also contribute to the transition toward chronic disease [5]. There is a complex interaction between allergen exposure, sensitization and subsequent disease development. It has been shown to be influenced by several factors, including genetic susceptibility, route of exposure, dose of allergen and in some cases by the structural characteristics of the allergen [6]. Populations and individuals, furthermore, are exposed to a mixture of several allergens, irritants and pollutants and their possible synergistic effects.
Fig. 32.1 A simple schematic representation of allergen sensitization. Dendritic cells are essential for priming and Th2 differentiation of naïve T-cells towards aeroallergens. Contamination of antigens with pattern-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) as well as locally produced damage-associated molecular patterns (DAMPs), such as uric acid and adenosine triphosphate (ATP), will augment an immune response.