Human development is separated into embryonic (before the onset of bone marrow function) and fetal periods, corresponding, respectively, to conception until approximately 2 months estimated gestational age (EGA), and to 2 months EGA until birth. The first trimester includes the entire embryonic period and the first stages of the fetal period. Histogenesis of all skin regions is initiated in the embryo, and differentiation of some of those tissues begins to occur [2]. The boundary between the first and second trimesters, at 3 months of age, is based only on fetal age and not on any remarkable changes in structure, composition or function of any region of the skin.
The second trimester includes many important events in skin development that can be correlated with changes in function and, at the same time that morphogenesis of new structures is initiated, there is terminal differentiation of others. During the third trimester, as far as we know, all parts of the skin are assembled and the functions of each of them are unfolding. The end of this period is not the final state of the skin, as there is significant reorganization of certain units of the skin (e.g. the vasculature), additions to the skin in volume (e.g. the dermal matrix) and functional maturation of many structures of the skin (e.g. nerves, sweat glands and stratum corneum) after the newborn faces the environment of the external world [3–6,7].
Other important times that should be recognized in skin development are the ages at which chorionic villus sampling, amniocentesis and fetal skin biopsy are performed for the purpose of evaluating the condition of a fetus at risk for a genetic skin disease. Fetal DNA can be extracted from chorionic villi sampled around 10 weeks EGA, amniotic fluid cells can be obtained at around 14–16 weeks EGA, and fetal skin can be sampled as early as 16 weeks EGA. More typically, the skin is biopsied at 19–21 weeks [8–10]. The older age is necessary when diseases of keratinization are in question.
This chapter is organized to describe specific periods, unique features and special events or processes of skin development using EGA, the age from the date of conception, to represent the age of the embryo or fetus in utero. In the literature gestational age is used interchangeably with menstrual age, which is calculated from the date of the last known menstrual period and thus records the timing of developmental events about 2 weeks later than EGA. Unanswered questions will be raised for the reader’s thoughtful consideration.
References
1 Holbrook KA, Odland GF. The fine structure of developing human epidermis: light, scanning and transmission electron microscopy of the periderm. J Invest Dermatol 1975;65:16–38.
2 Holbrook KA, Dale BA, Smith LT et al. Markers of adult skin expressed in the skin of the first trimester fetus. Curr Prob Dermatol 1987;16:94–108.
3 Holbrook KA. Structure and function of the developing human skin. In: Goldsmith LA (ed.) Physiology, Biochemistry and Molecular Biology of the Skin, 2nd edn. Oxford: Oxford University Press, 1991: 63–110.
4 Holbrook KA. Structural and biochemical organogenesis of skin and cutaneous appendages in the fetus and neonate. In: Polin RA, Fox WW (eds) Neonatal and Fetal Medicine Physiology and Pathophysiology. New York: Grune & Stratton, 1992: 527–51.
5 Holbrook KA, Wolff K. The structure and development of skin. In: Fitzpatrick TB, Eisen AZ, Wolff K et al. (eds) Dermatology in General Medicine, 6th edn. New York: McGraw-Hill, 1993: 97–144.
6 Holbrook KA, Sybert VP. Basic science. In: Schachner L, Hansen R (eds) Pediatric Dermatology, 2nd edn. New York: Churchill Livingstone, 1995.
7 Holbrook KA. A histologic comparison of infant and adult skin. In: Boisits E, Maibach HI (eds) Neonatal Skin: Structure and Function. New York: Marcel Dekker, 1982: 3–31.
8 Holbrook KA, Smith LT, Elias S. Prenatal diagnosis of genetic skin disease using fetal skin biopsy samples. Arch Dermatol 1993;129:1437–54.
9 Sybert VP, Holbrook KA, Levy M. Prenatal diagnosis of severe dermatologic diseases. Adv Dermatol 1992;7:179–209.
10 Sybert VP, Holbrook KA. Antenatal pathology of the skin. In: Claireaux AE, Reed GB (eds) Diseases of the Fetus and Newborn: Pathology, Radiology and Genetics. New York: Cockburn, Chapman & Hall, 1995: 755–68.
Embryonic Skin
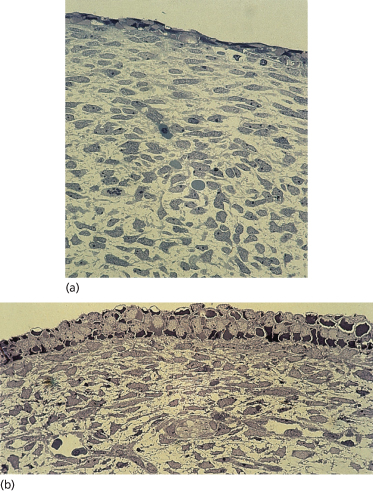
The primitive ectoderm of the developing blastocyst is established at 1 week EGA, and by 20–50 days EGA the major organs and organ systems of the human embryo are becoming established. The integumentary system exhibits characteristics of the skin at 30 days EGA, the earliest age at which specimens can be realistically obtained for study. Sampling of skin at this age is remarkable considering that the 6-week-old embryo has a crown–rump length of only 20 mm – no larger than a 20 pence piece (or a dime). Nonetheless, the epidermis, dermoepidermal junction (DEJ) and dermis are well delineated and the tissue is innervated and vascularized (Fig. 2.2). The boundary between the dermis and subcutaneous tissue is not clearly defined in all body sites, but in some regions these two zones are distinct from one another on the basis of a greater density of cells and matrix in the dermis compared with the hypodermis. The skin is closely associated with the underlying developing striated muscle or cartilage on the appendages. There is no morphological evidence that epidermal appendages have begun to form.
Fig. 2.2 (a) Tissue of the body wall of a 36-day EGA human embryo and (b) the skin from a 45-day EGA human embryo. Note the two-layered epidermis, dermis and subcutaneous tissue and the more linear orientation of dermal cells in contrast to the pleiomorphic shapes of the subcutaneous mesenchyme. In (b) note the periderm and basal cells of the epidermis, the closely associated fibroblastic cells in the dermis proximal to the epidermis and a nerve–vascular plane separating the dermis from the subcutaneous tissue (×200).
In most regions of the embryo, the epidermis is a simple, flat, two-layered epithelium consisting of basal and periderm cells (see below) (Figs 2.2 and 2.3). Both types of cells are mostly filled with glycogen, a molecule that is characteristic in the cytoplasm of developing and regenerating tissues, where it most likely serves as a source of energy [1] (Fig. 2.3). The nucleus is centrally located in periderm and basal cells, and the cytoplasmic organelles are sparse and distributed either around the nucleus or at the periphery of the cell (Fig. 2.3b). The structural characteristics of these cells therefore fail to reveal significant differences between the two layers or to verify that either layer is composed of keratinocytes. Evaluation of the structural proteins, however, indicates that both contain keratin intermediate filament proteins (Fig. 2.4), but different species [2,3], and some cell-surface molecules are unique to each layer [4]. The latter markers may reflect the differences in environments surrounding each layer.
Fig. 2.3 Transmission electron micrographs of the embryonic epidermis. In (a) note the glycogen (G)-filled basal (B) and periderm (P) layer cells. Desmosomes are evident between basal cells and between basal cells and periderm cells. The DEJ is flat and shows few sites of increased density, suggesting sites of desmosome formation. In (b) one periderm cell and portions of two basal cells are shown. Note the nature and disposition of cytoplasmic organelles within both cell types, the keratin filaments associated with desmosomes (arrow) and the microvilli extending from the periderm surface (a, ×11,525; b, ×25,000).
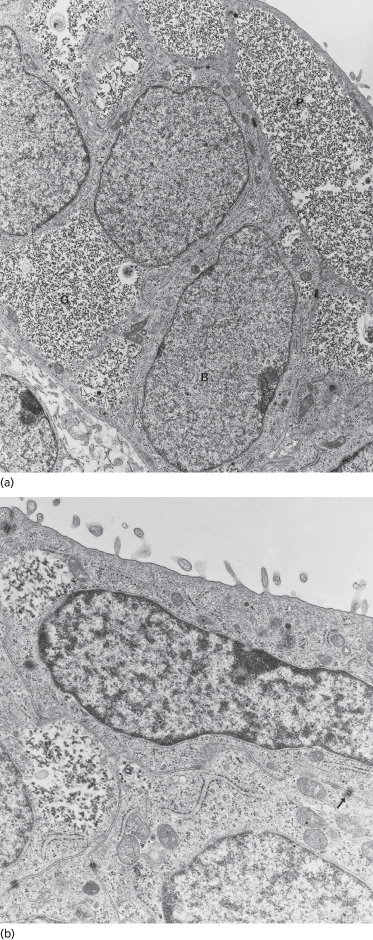
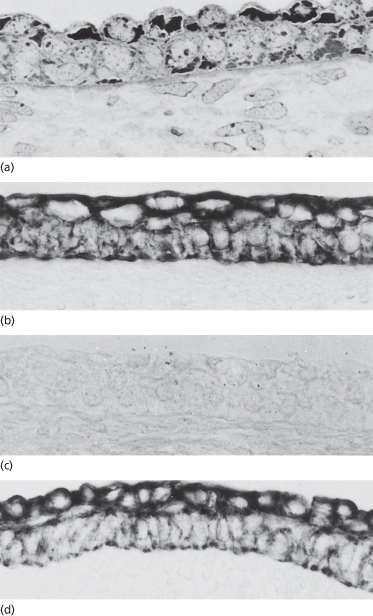
Fig. 2.4 Immunostained samples of (a) early (∼50-day EGA) and (b–d) later (∼60-day) human embryonic epidermis showing positive staining of both periderm and basal layers with the AE1 (a) and AE3 (d) monoclonal antibodies that recognize keratins. Both layers are negative when reacted with the AE2 (c) antibody, which recognizes the differentiation-specific keratins (×350).
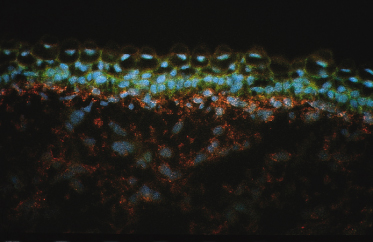
The columnar-shaped basal cells of the embryonic epidermis express the keratins that are characteristic of adult basal layer keratinocytes – K5 (58 kDa) and K14 (50 kDa) [2,3] – and additional keratin polypeptides – K19 (40 kDa) and K8 (52 kDa) – are specific to embryonic/fetal basal cells and periderm cells [2,3]. The latter keratins are not characteristic of normal adult basal keratinocytes, but they are identified in glandular and simple epithelial cells [5]. In contrast to the adult tissue, the filaments in fetal embryonic epidermis are dispersed in the cytoplasm or assembled in small, seemingly short, bundles that are associated primarily with desmosomes and hemidesmosomes (see Fig. 2.3b). Periderm cells and basal cells also differ in the expression of many growth factors, growth factor receptors (Fig. 2.5) [6,7], cell adhesion molecules [8] and other cytoplasmic and cell-surface molecules.
Fig. 2.5 Section of skin from a 78-day EGA human fetus showing differential expression of the A-chain of PDGF in the basal and intermediate cell layers (green) and an absence of staining in peridermal cells. The receptor for PDGFA, PDGFR-α (red), is expressed by cells in the dermis (×350).
About 15–19% of the basal cells incorporate label when viable tissue is incubated in culture medium containing [3H]-thymidine for 1 h, rinsed and then processed for autoradiography. This is a higher labelling index than the fetal or adult epidermis, which was recorded at 10% and 6.7% labelling, respectively, under the same conditions [9]. It is possible that only the basal layer is truly epidermis.
A thin, flattened layer of periderm cells covers the basal layer, with no apparent correspondence in number with the cells beneath it, i.e. one polygonal periderm cell lies above several basal cells. Microvilli project from the peridermal surface into the amniotic fluid (Figs 2.3b and 2.6). At least one keratin polypeptide expressed in periderm cells is different from those in the basal cells, K18 (45 kDa), although it is a marker for Merkel cells [10,11].
Fig. 2.6 Scanning electron micrograph of the surface of 55-day EGA embryonic skin from the surface of the developing foot. The layer of cells shown is the periderm. Note the microvilli and the variable size and shape of the cells (×1000).
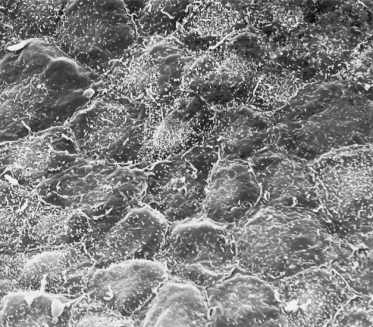
Two of the immigrant cells that are prominent in adult epidermis, melanocytes (neural crest in origin) and Langerhans cells, are present in the embryonic epidermis among basal cells and associated with the basement membrane. Sheets of embryonic epidermis immunostained with an antibody that recognizes melanocytes specifically (HMB-45, an inducible, cytoplasmic antigen common to melanoma and embryonic/fetal melanocytes [12,13]) show a remarkably high density (∼1000 cells/mm2) of these cells organized in a regular pattern of distribution (Fig. 2.7). They are dendritic as early as 50 days EGA in general body skin but there is no evidence of melanosomes in the cytoplasm [14]. Langerhans cells are recognized in embryonic skin as early as 42 days EGA on the basis of a reaction product for membrane-bound Mg2+ adenosine triphosphatase (ATPase) and histocompatibility locus antigen (HLA-DR) on the plasma membrane [15–17] and by their truncated or dendritic morphology (Fig. 2.8). They are probably derived from the yolk sac or fetal liver at this age because they are present in skin before the bone marrow begins to function. At 7 weeks EGA, the density of Langerhans cells is about 50 cells/mm2 [16,17].
Fig. 2.7 Embryonic skin from a 54-day EGA human embryo immunostained with the HBM-45 monoclonal antibody, which recognizes an antigen in the melanocyte. (a) Section of skin. Note the abundance and position of these cells within the two-layered epidermis. (b) Epidermal sheet. Note the density, spacing and dendritic morphology of these cells (a, ×350; b, ×25).
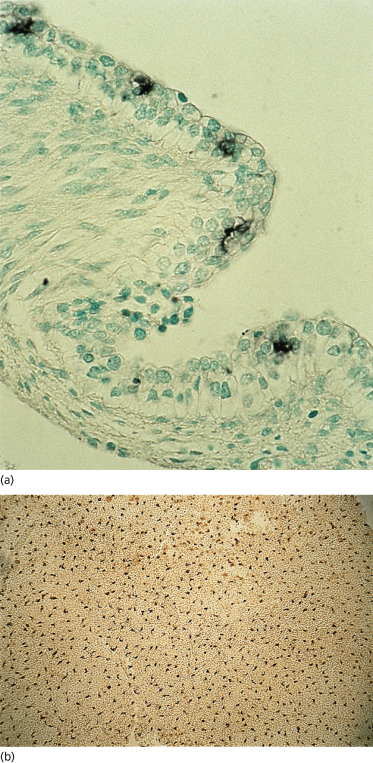
Fig. 2.8 Epidermal sheet from a 53-day EGA human embryo immunostained to recognize HLA-DR antigen in epidermal Langerhans cells (×400).
Micrograph courtesy of Dr Carolyn Foster.
The third immigrant cell, the Merkel cell, can be recognized in embryonic palmar skin as early as 55–60 days EGA (see Eccrine sweat gland formation) at a density of ∼130 cells/mm2 [10,18], using as a marker any one of the set of keratins expressed by Merkel cells (K8, K18, K10 and K20) [10,11,19–21]. K20 is the only keratin found exclusively in Merkel cells [21]. At this embryonic age, they are distributed randomly and in a suprabasal position. Merkel cells are neuroendocrine cells that were originally thought to function primarily as slow-adapting mechanoreceptors. More recently, studies that have catalogued soluble mediators produced by these cells, for example nerve growth factor (NGF) [22], suggest that it is likely that Merkel cells are targets for ingrowing nerve fibres or other cells such as the smooth muscle cells of the arrector pili muscle [23,24]. Their presence in selected sites of developing epidermal appendages (e.g. sweat glands and hair follicles) has also been suggested to stimulate or to correlate with active proliferation of the tissue. It is generally accepted that Merkel cells are derived from keratinocytes in situ [10,19,21,25,26].
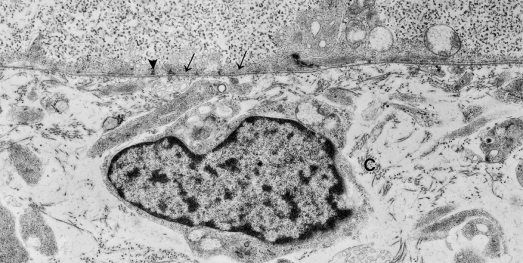
A continuous basal lamina (lamina densa) underlies the two-layered epidermis and defines, morphologically, one structural component of the basement membrane zone [27–29]. The basal lamina is patchy, however, in regions of the body where the epidermis may be only a single layer, for example superior to the spinal cord. The molecules and antigens characteristic of all basal laminae (type IV collagen, laminin, heparan sulphate proteoglycan, nidogen/entactin) are present in the earliest recognized basal lamina of the skin; skin-specific molecules are recognized later during the first trimester in accord with the more prominent development of the attachment structures (see below) [30,31]. A thin, mat-like layer of microfilaments lies just inside the basal plasma membrane of the basal cell keratinocytes (Fig. 2.9). It may reinforce this surface of the epidermis and add to the strength of the DEJ at this stage when the structural modifications associated with dermoepidermal adhesion (hemidesmosomes, anchoring filaments, anchoring fibrils) are rudimentary [32]. The same organization of filaments is observed in cultured keratinocytes, which do not typically form hemidesmosomes and anchoring fibrils in vivo, and in basal keratinocytes under pathological situations, such as junctional epidermolysis bullosa, in which the epidermis separates from the dermis.
Fig. 2.9 Enlarged view of the DEJ of human embryonic epidermis showing the microfilament network within the basal epidermal cell (arrows), sites where desmosomes are forming (arrowheads) and the lamina densa. Note collagen fibrils (C) surrounding the dermal fibroblastic cells (×11,625).
The antigens associated with the attachment structures (laminin 5/epiligrin/kalinin and 19 DEJ-1 for hemidesmosomes and anchoring filaments [33–36]; type VII collagen for anchoring fibrils [37]) are not seen by light microscopic immunostaining methods until early in the fetal period. It is likely, however, that keratinocytes begin to synthesize these proteins in the embryonic period but that the methods used for detection are not sensitive enough to demonstrate their low levels of expression. The dermoepidermal boundary is flat in the embryonic skin (Figs 2.2, 2.3 and 2.9) and thus presents a limited surface area for nutrients to traverse between the dermis and the epidermis. This may be relatively less important in the developing skin than in infant and adult skin because the dermis is thin and the small, dispersed bundles of dermal matrix proteins and the hydrated condition of the interstitial matrix permit more rapid diffusion of substances than the mature skin.
The dermis in the embryo is highly cellular (Figs 2.2 and 2.10), but it also contains the extracellular fibrous matrix proteins, types I, III, V and VI interstitial collagens, characteristic of adult dermis [32,38–40,41–45]. Small bundles of collagen accumulate in a thin, dense layer, called the reticular lamina, immediately beneath the dermoepidermal interface (Figs 2.2b, 2.5 and 2.9). They are also dispersed throughout the dermis in varying densities according to the collagen type and age of the embryo. Types I, III and VI collagen are distributed uniformly throughout the dermis whereas type V collagen is concentrated primarily along basement membranes (at the DEJ and around blood vessels) and surrounding cells (Fig. 2.11). Fibre bundles within the interstitial spaces are widely dispersed by a hydrated, hyaluronic acid-rich proteoglycan matrix [46–48] (Fig. 2.12). The fluidity of the matrix at this stage permits migration of mesenchymal cells to sites of active tissue morphogenesis.
Fig. 2.10 Transmission (a) and scanning (b) electron micrographs of the embryonic dermis at 48 days EGA beginning at the DEJ. The matrix is less evident in the sectioned sample (a) than in the whole-mount specimen (b) (a, ×4500; b, ×1500).
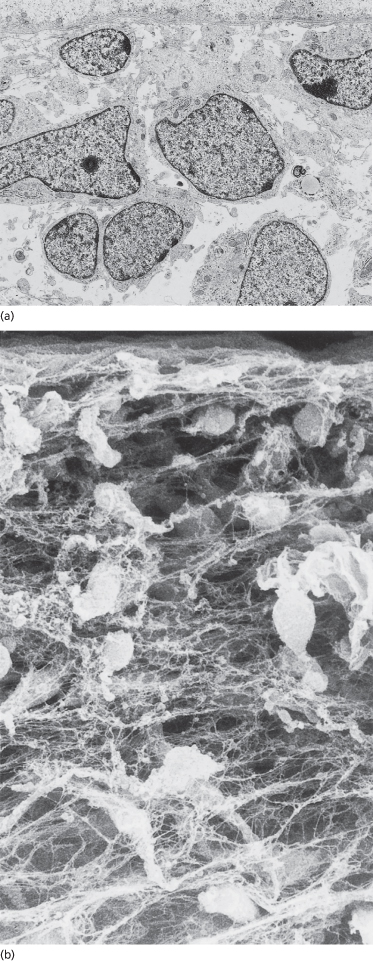
Fig. 2.11 Samples of embryonic skin immunostained with antibodies that recognize type I (a), III (b), V (c) and VI (d) collagens. Note that all of the collagens are concentrated beneath the DEJ but types III and V, especially, are found in association with all basement membranes. Types I, III and VI are found in the matrix throughout the dermal and subcutaneous tissue (a–c, ×150; d, ×300).
Immunostaining courtesy of Dr Lynne T. Smith.
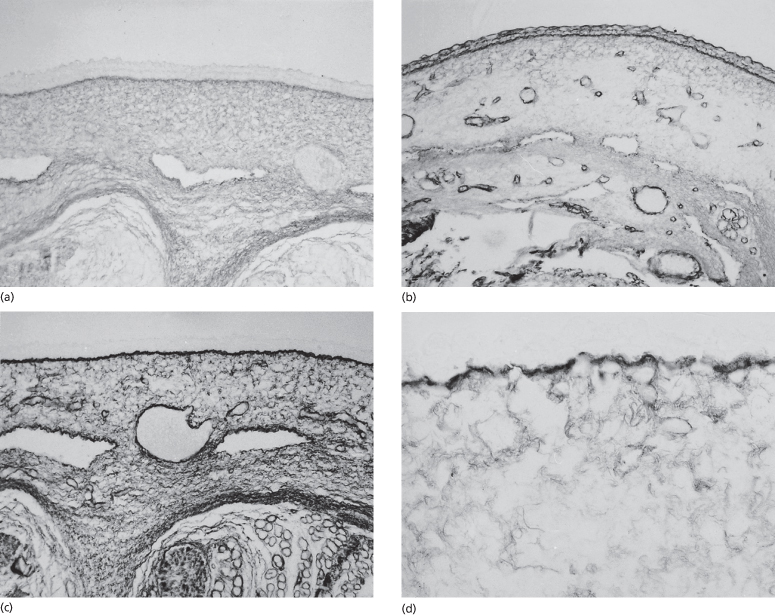
Fig. 2.12 Section of the body wall from a 57-day EGA embryo treated with the Alcian blue/periodic acid–Schiff (PAS) histochemical stains. The bright pink staining of the epidermis (glycogen) and DEJ (glycoproteins) indicates a PAS-positive reaction. The blue dermis reflects the high content of hyaluronic acid. The dermal–subcutaneous boundary is marked by a transition to a lighter slightly purple reaction indicating more of the collagen–glycosaminoglycan complex (×300).
Immunohistochemistry courtesy of Dr Richard Frederickson.
A broader zone of sulphated proteoglycan-rich matrix, called the compact mesenchyme, is delineated beneath the epidermis on the basis of its rich concentration of cells that express growth factor receptors – the platelet-derived growth factor receptor β (PDGFR-β) and PDGFR-α (see Fig. 2.5), nerve growth factor receptor (NGFR) – and cell adhesion molecules (e.g. neural cell adhesion molecule, NCAM) [49,50]. Evidence from the skin of non-human species during development has shown enlargement of the composition of growth factors and receptors and adhesion molecules that are included in this dermal zone (reviewed in refs [49] and [51–53]). The compact mesenchyme may be involved in the exchange of signals between the epidermis and dermis and may be very important in stimulating the onset of appendage formation. Many of the growth factors that correspond to the receptors on the mesenchymal cells are produced by cells of the developing epidermis (e.g. PDGF-AA, PDGF-BB and NGF) (Fig. 2.5). The compact mesenchyme may also be the earliest evidence of a papillary dermis. In the adult, the modified composition and structure of the papillary dermis probably reflects molecular interactions between the epidermal and dermal cells, similar to the situation of the compact mesenchyme.
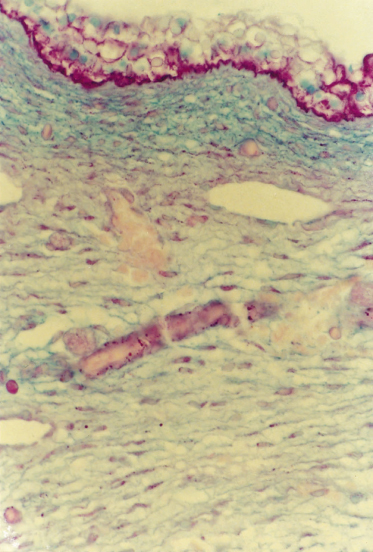
Elastic fibres per se are not formed in the embryonic skin, but fibrillin (the microfibrils of elastic fibres) (Fig. 2.13) and elastin proteins of the elastic fibre can be identified immunohistochemically [32,38–42,44] and microfibrils can be seen by electron microscopy [32].
Fig. 2.13 Section of skin from a 57-day EGA human embryo immunostained with an antifibrillin antibody. Note staining throughout the dermis (×200).
Immunostaining courtesy of Dr Lynne T. Smith.

Fine nerve fibres and capillaries are present within the compact mesenchyme and deeper dermis (Fig. 2.14a), and large nerve trunks and vessels are readily apparent in the subcutaneous region. Reconstructions of vessels from serial sections of developing first-trimester skin have shown that the the basic pattern of cutaneous vasculature is established in the first trimester [54]. New vessels presumably both form de novo from dermal mesenchyme and sprout from deeper, established vessels through a process that includes endothelial cell migration, capillary budding and vessel remodelling [55]. The events and mechanisms of these processes have not been explored beyond the morphological descriptions and antigenic characterization of the endothelial cells at various ages during development [55,56]. Pieces of full-thickness skin and sections of skin immunostained with an antibody that demonstrates all cutaneous nerves (protein gene product 9.5 or PGP 9.5) [57,58] reveal finely beaded nerve filaments distributed in an impressive density in the subepidermal region and in association with blood vessels (Fig. 2.14b and c). The number of fibres recognized by this antibody increases during development as the fibres become organized in networks throughout the dermis and in relation to developing epidermal appendages [59]. At 7 weeks EGA, a few calcitonin gene-related product (CGRP)-immunopositive fibres, denoting sensory fibres, are also evident [59], but autonomic nerves are not yet recognized in the skin. Staining the tissue with the p75 low-affinity NGFR antibody also reveals the patterns of nerve fibres and specific concentrations of mesenchymal cells (e.g. around developing hair follicles; see below) [60]. Both nerves and vessels are visible in stained, full-thickness samples of the nearly transparent skin (Fig. 2.15).
Fig. 2.14 Sections of human embryonic skin at 42 days EGA (a) and 59 days EGA (b) immunostained with PGP 9.5, which recognizes all cutaneous nerves, and of a sample of 52-day EGA embryonic skin (c) immunostained with p75 antibody, which recognizes the low-affinity NGFR. Note the large nerve trunks deep in the subcutaneous tissue (a), the significant density of the fine fibres in the tangential section of the dermis of (b) and the distribution of both nerves and vessels (c) (a, ×100; b, ×200; c, ×200).
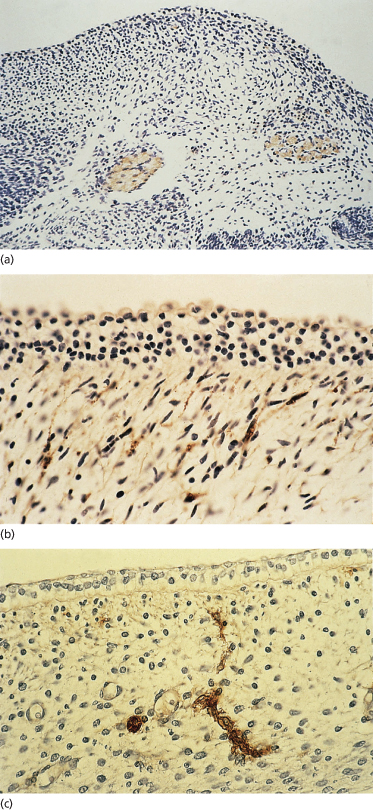
Fig. 2.15 Nerves and vessels in the skin of a 79-day EGA human fetus immunostained with an anti-neurofilament antibody. Note the positively stained nerve network, the immunopositive cells (presumably Merkel cells) and the vascular network (clear) (×25).
Immunostaining courtesy of Dr Mark Bressler.
References
1 Sharp F. A quantitative study of the glycogen content of human fetal skin in the first trimester. J Obstet Gynaecol Br Commonw 1971;78:981–6.
2 Dale BA, Holbrook KA, Kimball JR et al. Expression of the epidermal keratins and filaggrin during fetal human development. J Cell Biol 1985;101:1257–69.
3 Moll R, Moll I, Wiest W. Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation 1983;23:170–8.
4 Dabelsteen E, Holbrook KA, Clausen H et al. Cell surface carbohydrate changes during embryonic and fetal skin development. J Invest Dermatol 1986;87:81–5.
5 Moll R, Franke WW, Schiller DL. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31:11–24.
6 Piepkorn M, Underwood RA, Henneman C et al. Expression of amphiregulin is regulated in cultured human keratinocytes and in developing fetal skin. J Invest Dermatol 1996;105:802–9.
7 Nanney LB, Stoscheck CM, King LE et al. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol 1990; 94: 742–8.
8 Fujita M, Furukawa F, Fujii K et al. Expression of cadherin molecules during human skin development: morphogenesis of epidermis, hair follicles and eccrine sweat ducts. Arch Dermatol Res 1992;284:159–66.
9 Bickenbach JR, Holbrook KA. Label retaining cells (LRCs) in human embryonic and fetal epidermis. J Invest Dermatol 1986;88:42–6.
10 Moll R, Moll I, Franke W. Identification of Merkel cells in human skin by specific cytokeratin antibodies: changes in cell density and distribution in fetal and adult plantar epidermis. Differentiation 1984;28:136–54.
11 Moll R, Moll I. Early development of human Merkel cells. Exp Dermatol 1992;1:180–4.
12 Gown AM, Vogel AM, Hoak D et al. Monoclonal antibodies specific for melanocytic tumors distinguish populations of melanocytes. Am J Pathol 1986;123:195–203.
13 Smoller BR, Hsu A, Krueger J. HMB-45 monoclonal antibody recognizes an inducible and reversible melanocyte cytoplasmic protein. J Cutan Pathol 1991;8:315–22.
14 Holbrook KA, Underwood RA, Vogel AM et al. The appearance, density and distribution of melanocytes in human embryonic and fetal skin revealed by the anti-melanoma monoclonal antibody, HMB-45. Anat Embryol 1989;180:443–55.
15 Foster CA, Holbrook KA, Farr AG. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol 1986;86:240–3.
16 Drijkoningen M, DeWolf-Peeters C, VanDerSteen K et al. Epidermal Langerhans cells and dermal dendritic cells in human fetal and neonatal skin: an immunohistochemical study. Pediatr Dermatol 1987;4:11–17.
17 Foster CA, Holbrook KA. Ontogeny of Langerhans cells in human embryonic and fetal skin: cell densities and phenotypic expression relative to epidermal growth. Am J Anat 1989;84:157–64.
18 Kim D-G, Holbrook KA. The appearance, density and distribution of Merkel cells in human embryonic and fetal skin: their relation to sweat gland and hair follicle development. J Invest Dermatol 1995;104:411–16.
19 Moll I, Moll R, Franke W. Formation of epidermal and dermal Merkel cells during human fetal skin development. J Invest Dermatol 1986;87:779–87.
20 Moll R, Löwe A, Laufer J et al. Cytokeratin 20 in human carcinomas: a new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 1992;140:427–47.
21 Moll I, Kuhn C, Moll R. Cytokeratin 20 is a general marker of cutaneous Merkel cells while certain neuronal proteins are absent. J Invest Dermatol 1995;104:910–15.
22 Vos P, Stark F, Pittman RN. Merkel cells in vitro: production of nerve growth factor and selective interactions with sensory neurons. Dev Biol 1991;144:281–300.
23 Narisawa Y, Hashimoto K, Nakamura Y et al. A high concentration of Merkel cells in the bulge prior to the attachment of the arrector pili muscle and the formation of the perifollicular nerve plexus in human fetal skin. Arch Dermatol Res 1993;285:261–8.
24 Moore SJ, Munger BL. The early ontogeny of the afferent nerves and papillary ridges in human digital and glabrous skin. Dev Brain Res 1989;48:119–41.
25 Moll I, Lane AT, Franke WW et al. Intraepidermal formation of Merkel cells in xenografts of human fetal skin. J Invest Dermatol 1990;94:359–64.
26 Narisawa Y, Hashimoto K. Immunohistochemical distribution of nerve–Merkel cell complex in fetal human skin. J Dermatol Sci 1991;2:361–70.
27 Marinkovich MP, Keene DR, Rimberg CL. Cellular origin of the dermal–epidermal basement membrane. Dev Dyn 1993;196:255–67.
28 Christiano AM, Uitto J. Molecular complexity of the cutaneous basement membrane zone: revelations through the paradigms of epidermolysis bullosa. Exp Dermatol 1996;5:1–11.
29 Uitto J, Pulkkinen L. Molecular complexity of the cutaneous basement membrane zone. Mol Biol Rep 1996;23:35–46.
30 Fine JD, Smith LT, Holbrook KA et al. The appearance of four basement membrane zone antigens in developing human fetal skin. J Invest Dermatol 1984;83:66–9.
31 Smith LT, Sakai LY, Burgeson RE et al. Ontogeny of structural components at the dermal–epidermal junction in human embryonic and fetal skin: the appearance of anchoring fibrils and type VII collagen. J Invest Dermatol 1988;90:480–5.
32 Holbrook KA. Structure and function of the developing human skin. In: Goldsmith LA (ed.) Physiology, Biochemistry and Molecular Biology of the Skin, 2nd edn. Oxford: Oxford University Press, 1991: 63–110.
33 Hopkinson SB, Riddelle KS, Jones JCR. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol 1992;99:264–70.
34 Ishiko A, Shimizu H, Kikuchi A et al. Human autoantibodies against the 230 kD bullous pemphigoid antigen (BPAG1) bind only to the intracellular domain of hemidesmosome, whereas those against the 180 kD bullous pemphigoid antigen (BPAG2) bind along the plasma membrane of hemidesmosome in normal human and swine skin. J Clin Invest 1993;91:1608–15.
35 Verrando P, Blanchet-Bardon C, Pisani A et al. Monoclonal antibody GB3 defines a widespread defect of several basement membranes and a keratinocyte dysfunction in patients with lethal junctional epidermolysis bullosa. Lab Invest 1991;64:85–92.
36 Fine J-D, Horiguchi Y, Couchman JR. 19-DEJ-1, a hemidesmosomal–anchoring filament complex associated monoclonal antibody: definition of a new skin basement membrane antigenic defect in junctional and dystrophic epidermolysis bullosa. Arch Dermatol 1989;125:520–3.
37 Burgeson RE. Type VII collagen, anchoring fibrils and epidermolysis bullosa. J Invest Dermatol 1993;101:252–5.
38 Holbrook KA. Structural and biochemical organogenesis of skin and cutaneous appendages in the fetus and neonate. In: Polin RA, Fox WW (eds) Neonatal and Fetal Medicine Physiology and Pathophysiology. New York: Grune & Stratton, 1992: 527–51.
39 Holbrook KA, Wolff K. The structure and development of skin. In: Fitzpatrick TB, Eisen AZ, Wolff K et al. (eds) Dermatology in General Medicine, 6th edn. New York: McGraw-Hill, 1993: 97–144.
40 Holbrook KA, Sybert VP. Basic science. In: Schachner L, Hansen R (eds) Pediatric Dermatology, 2nd edn. New York: Churchill Livingstone, 1995.
41 Smith LT, Holbrook KA, Byers PH. Structure of the dermal matrix during development and in the adult. J Invest Dermatol 1982;79:93S–104S.
42 Smith LT, Holbrook KA. Development of dermal connective tissue in human embryonic and fetal skin. Scan Electron Microsc 1982:1745–51.
43 Smith LT, Holbrook KA, Madri JA. Collagens types I, III and V in human embryonic and fetal skin. Am J Anat 1986;175:507–22.
44 Smith LT, Holbrook KA. Embryogenesis of the dermis. Pediatr Dermatol 1986;3:271–80.
45 Smith LT. Patterns of type VI collagen compared to types I, III, and V collagen in human embryonic and fetal skin and in fetal skin-derived cell cultures. Matrix Biol 1994:14:159–70.
46 Breen M, Weinstein HG, Johnson RL et al. Acid glycosaminoglycans in human skin during fetal development and in adult life. Biochim Biophys Acta 1970;201:54–60.
47 Varma RS, Varma R. Glycosaminoglycans and proteoglycans of skin. In: Varma RS, Varma R (eds) Glycosaminoglycans and Proteoglycans in Physiological and Pathological Processes of Body Systems. Basle: Karger, 1982: 151–64.
48 Widdowson EM. Changes in the extracellular compartment of muscle and skin during normal and retarded development. Bibl Nutr Dieta 1969;13:60–8.
49 Holbrook KA, Smith LT, Kaplan ED et al. The expression of morphogens during human follicle development in vivo and a model for studying follicle morphogenesis in vitro. J Invest Dermatol 1993;101:39S–49S.
50 Kaplan ED, Holbrook KA. Dynamic expression patterns of tenascin, proteoglycans and cell adhesion molecules during human hair follicle morphogenesis. Dev Dyn 1994;199:141–55.
51 Chuong C-M, Widelitz RB, Jiang T-X. Adhesion molecules and homeoproteins in the phenotypic determination of skin appendages. J Invest Dermatol 1993;101:10S–15S.
52 Chuong C-M, Widelitz RB, Ting-Berreth S et al. Early events during avian skin appendage regeneration: dependence on epithelial–mesenchymal interaction and order of molecular reappearance. J Invest Dermatol 1996;107:639–46.
53 Widelitz RB, Jiang T-X, Noveen A et al. FGF induces new feather buds from developing avian skin. J Invest Dermatol 1996;107:797–803.
54 Johnson CL, Holbrook KA. Development of human embryonic and fetal dermal vasculature. J Invest Dermatol 1989;93:10S–17S.
55 Karelina TV, Goldberg GI, Eisen AZ. Matrix metalloproteinases in blood vessel development in human fetal skin and in cutaneous tumors. J Invest Dermatol 1995;105:411–17.
56 McGowan KA, Bauer EA, Smith LT. Localization of type I human skin collagenase in developing embryonic and fetal skin. J Invest Dermatol 1994;102:951–7.
57 Dalsgaard CJ, Rydh M, Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry 1989;92:385–9.
58 Gulbenkain S, Wharton J, Polak JM. The visualization of cardiovascular innervation in the guinea pig using an antibody to protein gene-product 9.5. J Autonom Nerv Syst 1987;18:235–47.
59 Terenghi G, Sundaresan M, Moscoso G et al. Neuropeptides and a neuronal marker in cutaneous innervation during human foetal development. J Comp Neurol 1993;328:595–603.
60 Holbrook KA, Bothwell MA, Schatteman G et al. Nerve growth factor receptor labelling defines developing nerve networks and stains follicle connective tissue cells in human embryonic and fetal skin. J Invest Dermatol 1988;90:609A.
Embryonic–Fetal Transition
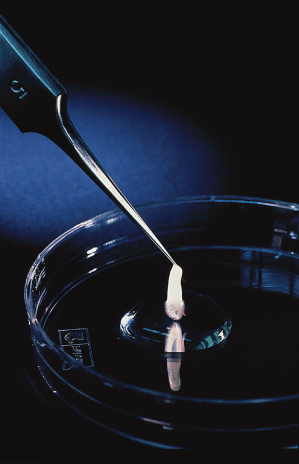
The most remarkable time in skin development is the embryonic–fetal transition, which occurs at approximately 2 months’ gestation, when the embryo measures about 31 mm in length (crown–rump), weighs about 2.5 g and has a humanoid appearance. The skin and the underlying tissues of the body wall are translucent, revealing the ribs and solid organs. The skin has a mucoid quality when removed from the body (Fig. 2.16). In spite of this structural simplicity, with few exceptions, the cells in the skin begin to express nearly every characteristic of adult skin. The 2-month age is thus identified as an important landmark in skin development (see Fig. 2.1).
Fig. 2.16 Sample of human fetal skin of 80-days EGA held at the tips of a forceps and demonstrating the mucoid quality of the tissue.
It is unlikely that the changes occurring around this period are triggered simultaneously and more probable that they unfold over a period of time. What initiates these changes in all regions of the skin is unclear, but the results are dramatically evident when the tissues are assayed for expression of the markers of differentiation – as we understand differentiation in the adult skin – that were not expressed in the embryonic skin. It is likely that a constellation of genes is triggered, but in response to what signals? Since this period of development appears to be very much concerned with the establishment of adult characteristics of the skin, it is perhaps also a stage that is vulnerable to errors in development. The embryonic–fetal transition needs to be studied with modern techniques of molecular biology to gain a better understanding of the coordinated onset of expression of new properties of all regions of the skin and the introduction of another set of morphogenetic processes that result in the formation of skin appendages (hair follicles, sweat glands, teeth and nails).
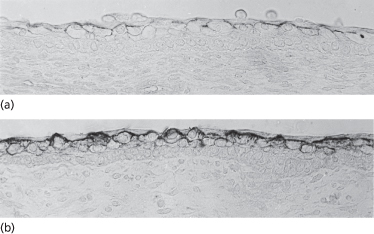
The most apparent change in the skin that is recognized at the embryonic–fetal transition is stratification of the epidermis from two to three cell layers (Fig. 2.17a and b). An intermediate cell layer is added as the product of basal cell mitoses. Basal cells divide asynchronously to produce an epidermis that, initially, remains two-layered at some sites and at others become three layers thick.
Fig. 2.17 Skin from a human fetus of approximately 70–89 days EGA shown at the light (a) and electron (b) microscopic levels. Note the intermediate layer of cells between basal and periderm epidermal layers, the distinction between dermis and subcutaneous tissue based on differences in the orientation of fibroblastic cells, the density of collagenous matrix and the subcutaneous vascular plane. Small nerves (n) and capillaries (c) are evident in the dermis. Segments of melanocytes (M) are evident within the basal layer and collagen is accumulated beneath the DEJ (×3675).

Intermediate cells are both similar to and distinct from basal and periderm cells. Keratins are more abundant and distributed in a more specific distribution than the cells in the basal and periderm layers; small bundles of keratin filaments associated with desmosomes outline the boundaries of the intermediate cells (Fig. 2.17b). The expression of the major keratin pairs in both basal- and intermediate-layer keratinocytes in the early fetal epidermis is now identical to the expression of the keratins in the fully keratinized adult epidermis. The K5 and K14 basal cell keratins are downregulated in the intermediate cells and a new keratin pair, K1 (56.5 kDa) and K10 (67 kDa), the high-molecular-weight differentiation-specific keratins, is synthesized [1,2] (Fig. 2.18). Other markers of keratinocyte differentiation (e.g. pemphigus antigen [3], cornified cell envelope proteins [4,5], blood group antigens [6] and cell-surface glycoproteins [7]; also reviewed in refs [8–11]) are also expressed in the cytoplasm or on the surface of intermediate layer cells. Like the periderm and basal cells, the first intermediate cells still contain glycogen as the primary cytoplasmic component (Fig. 2.17a and b). Thus, at this stage, when the epidermis is only a few cell layers thick, and has few similarities in morphology to adult epidermis, it nonetheless possesses all of the keratins and many of the other markers that are typical of the epidermis throughout life. It is important to understand this because it indicates that genetic diseases that involve mutations in keratin proteins have the potential of being expressed, and recognized, as early as the first trimester in development.
Fig. 2.18 Keratin filaments in the cells of the intermediate layer of 77-day EGA fetal skin stain positively with the AE-2 monoclonal antibody, which recognizes the K1 and K10 differentiation-specific keratins (a). The reaction pattern is even stronger when a second intermediate layer is added at the beginning of the second trimester (b) (a, ×120; b, ×120).
Initially, the keratinocytes of both basal and intermediate cell layers express epidermal growth factor (EGF) receptors [12] (Fig. 2.19), respond to EGF and retain the ability to proliferate [13,14]. Near the end of the first trimester, however, the proliferative cells become restricted primarily, if not exclusively, to the basal layer [13,14]; only the basal cells express P-cadherin [24], a marker of proliferative ability. Basal cells change in morphology and cell-surface properties after stratification. A greater volume of the cytoplasm is occupied by organelles and keratin filaments than with glycogen, and cell-surface carbohydrates that correlate with stratification and differentiation are differentially expressed by cells of the basal and intermediate layers [7,16]. Selected basal- and intermediate-layer keratinocytes participate in the formation of the epidermal appendages: the pilosebaceous structures, nails and teeth and the eccrine sweat glands in thick skin. The morphogenesis of these structures will be taken up in a separate section of this chapter (see below).
Fig. 2.19 Skin from a 72-day EGA fetus immunoreacted with an anti-epidermal growth factor receptor showing a reaction pattern on the membranes of basal and intermediate layer cells and on the basal and lateral borders of periderm cells (×120).
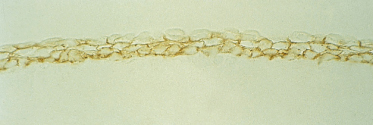
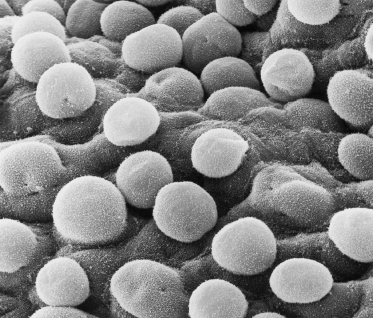
The cells of the periderm increase in size and begin to develop microvilli-covered blebs that extend from the outermost surface of the cell into the amniotic cavity (Fig. 2.20). It is likely that these modifications to increase cell-surface area have functional significance, but none has been specifically documented for this cell type. Function is implied only from structure (see below). The molecular species of keratins remain the same as they were in the embryonic periderm cells, but the cells lose their ability to divide and to express P-cadherin [13,15].
Fig. 2.20 Scanning electron micrograph of the periderm of a 60- to 70-day EGA human fetus showing the blebs and microvilli that modify the amniotic surface (×8000).
Because the epidermal cells that are located in the more superficial layers express differentiation-related antigens, it is appealing to link stratification and the onset of differentiation. There must be more processes involved, however, than simply the addition of cell layers because embryonic skin maintained in suspension organ culture stratifies to become several layers thick but will not differentiate in the manner characteristic of early fetal skin in vivo [17,18]. It remains unclear how stratification and the onset of expression of markers of differentiation in the epidermis (and the DEJ) are tied mechanistically.
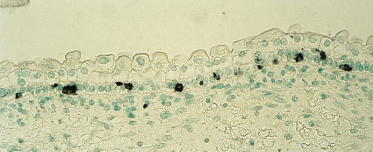
Melanocytes are easily recognized in sections of fetal epidermis at 8 weeks EGA by their position along the basement membrane, dense cytoplasm, an absence of glycogen and a heterochromatic nucleus [19]. Around 80 days EGA, they are present in the epidermis in maximal density (∼3000 cells/mm2) [19] compared with all other stages of skin development, and in a non-random distribution among cells of the basal layer (Fig. 2.21). The numbers decrease towards birth [20], then continue to decline over the decades of postnatal life.
Fig. 2.21 Section of skin from an 82-day EGA fetus immunostained with the HMB-45 antibody, which recognizes melanocytes. Note the high density of the cells and their position within the basal epidermal layer (×200).
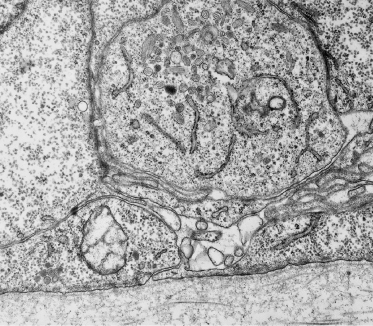
The high numbers of melanocytes around the embryonic–fetal transition may reflect the fact that these cells arrive early in the skin, proliferate and remain close together before there is substantial growth of the fetus. The labelling index of keratinocytes is also high [13] at this stage, suggesting that the paracrine interactions between melanocytes and keratinocytes that occur in the adult skin may be established early in development [19]. Melanosomes are recognized late in the third month of development (Fig. 2.22) and show some evidence of pigment formation in selected sites of the body [21,22].
Fig. 2.22 Section through late embryonic–early fetal skin showing developing melanosomes in a melanocyte positioned between keratinocytes in the basal layer. The early age of the tissue is confirmed by the immature structure of the DEJ (×25,000).
The importance of understanding the density of melanocytes and the onset of pigment synthesis relates to appropriate fetal skin sampling for the prenatal diagnosis of tyrosinase-negative oculocutaneous albinism [23]. Samples with a low density of cells are apt to have few cells available for examination in thick or thin sections. In such cases, it is possible to adopt methods to amplify the synthesis of melanin (dihydroxyphenylalanine, or DOPA, reaction) in the tissue samples [24], otherwise biopsies must be obtained at the time when and site where melanocytes are concentrated selectively (e.g. the bulb of the hair follicle at the bulbous hair peg stage).
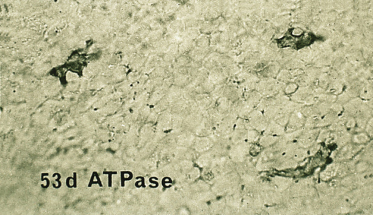
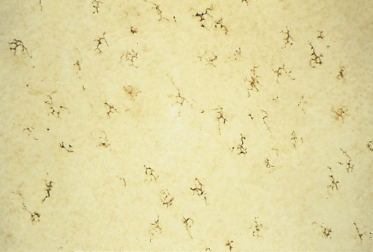
Langerhans cells are also more abundant in the epidermis at this stage (∼50/mm2) [25,26]. Unlike melanocytes, which migrate into the epidermis only during the embryonic period, the bone marrow-derived Langerhans cells migrate into the epidermis continually throughout life; their numbers do not increase significantly, however, until the third trimester and after birth [25,26]. Langerhans cells at 80 days EGA are highly dendritic (Fig. 2.23), begin to express CD1a at the surface [25–27] and develop Birbeck granules in the cytoplasm, suggesting that they may be capable of processing and presenting antigen in utero. The number of cells that are HLA-DR positive is significantly greater than the number that express CD1a at this stage; however, by about 13 weeks EGA Langerhans cells seem to express both markers with reasonable consistency.
Fig. 2.23 Epidermal sheet from an 80-day EGA fetus reacted to demonstrate ATPase. Note the regular distribution and density of these highly dendritic cells (×120).
By the end of the first trimester, Merkel cells are located along the primary epidermal ridges of palmar skin in regular alignment relative to the sites of origin of the sweat duct primordia (see below) and at a maximum density of ∼1400) cells/mm2 [28,29] (Fig. 2.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








