References
1 Marghoob AA, Scope A. The complexity of diagnosing melanoma. J Invest Dermatol 2009;129(1):11–13.
2 Binder M, Puespoeck-Schwarz M, Steiner A et al. Epiluminescence microscopy of small pigmented skin lesions: short-term formal training improves the diagnostic performance of dermatologists. J Am Acad Dermatol 1997;36:197–202.
3 Paganelli G, Soyer HP, Argenziano G et al. Diagnosis of pigmented skin lesions by dermoscopy: web-based training improves diagnostic performance of non-experts. Br J Dermatol 2003;148:698–702.
4 Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet 2002;3:159–65.
5 Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol 2001;137(10):1361–3.
6 Pan Y, Gareau DS, Scope A, Rajadhyaksha M, Mullani NA, Marghoob AA. Polarized and nonpolarized dermoscopy: the explanation for the observed differences. Arch Dermatol 2008;144(6):828–9.
7 Benvenuto-Andrade C, Dusza SW, Agero AL et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol 2007;143(3):329–38.
8 Yadav S, Vossaert KA, Kopf AW, Silverman M, Grin-Jorgensen C. Histopathologic correlates of structures seen on dermoscopy (epiluminescence microscopy). Am J Dermatopathol 1993;15(4):297–305.
9 Rezze GG, Scramim AP, Neves RI, Landman G. Structural correlations between dermoscopic features of cutaneous melanomas and histopathology using transverse sections. Am J Dermatopathol 2006;28(1):13–20.
10 Massi D, de Giorgi V, Soyer HP. Histopathologic correlates of dermoscopic criteria. Dermatol Clin 2001;19(2):259–68.
11 Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol 2001;137(12):1583–9.
12 Benvenuto C, Marghoob AA. Ten reasons why dermoscopy is beneficial for the evaluation of skin lesions. Expert Rev Dermatol 2006;1(3):1–6.
Congenital Melanocytic naevi
The presence of congenital melanocytic naevi (CMN) is determined in utero. Depending on a multitude of factors including timing of proliferation, senescence, melanin synthesis and melanin transfer, these naevi may be visible at birth or become apparent months to years thereafter. The aforementioned factors probably also exert an influence on the clinical morphology manifested by the CMN. Although the majority of senescent CMN will not change significantly over time, some may develop focal changes, the most feared of which is the development of melanoma.
The most widely used classification of CMN divides lesions into three categories based on size: small (<1.5 cm), medium (1.5–19.9 cm), and large (≥20 cm). While melanoma may develop in association with any CMN irrespective of size, the risk for developing melanoma appears to be proportional to the size of the CMN [1]. The location and age of onset of melanoma also appear to differ between small to medium and large CMN. Melanomas developing in smaller CMN tend to appear after puberty and are more likely to be located at the dermoepidermal junction and at the peripheral edge of the CMN. In contrast, melanomas occurring in large CMN develop earlier in life and are frequently located below the dermoepidermal junction.
Current management options for CMN include close clinical follow-up with or without baseline photographs and/or prophylactic surgical excision as feasible [2]. Most physicians, however, are electing to follow CMN, especially those that have a homogeneous clinical appearance and are small to medium in size, by periodic follow-up examinations. Dermoscopy has improved our ability to clinically monitor CMN [3]. Knowledge of the dermoscopic structures and patterns common to CMN can aid physicians in following these lesions and recognizing aberrancy that may be suggestive of melanoma. In other words, if the dermoscopic pattern does not conform to one of the commonly encountered CMN patterns or if focal atypical dermoscopic structural changes are observed, then a biopsy or an excision may be warranted. It is important to acknowledge that although most small and medium CMN are fairly homogeneous both clinically and dermoscopically, large CMN are often heterogeneous, displaying multiple islands of colour and irregular topography. However, each ‘island’ within the large CMN tends to be fairly homogeneous in its appearance.
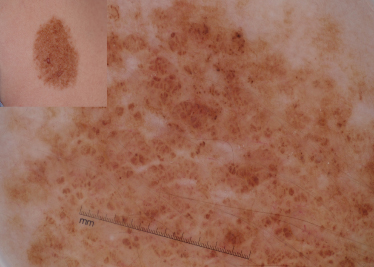
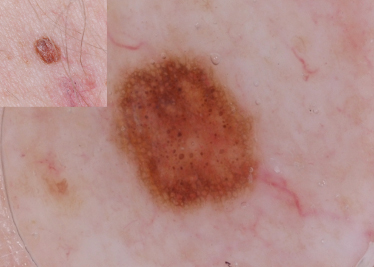
Dermoscopic evaluation of a CMN begins with the observation of dermoscopic structures (Table 185.2). A reticular network describes a honeycomb-like network of brown-black pigment in which pigmented lines correspond to hypermelanotic rete ridges and the open holes correspond to the suprapapillary plate or dermal papillae (Fig. 185.2) [4,5]. A reticular network, as seen in CMN, is characterized by its:
- quality, which can be fine and/or thick
- distribution, in which the network can be present homogeneously throughout the lesion, or present focally (patchy), or may be present only at the periphery
- specific type, linear network fragments or branched streaks that resemble hyphal elements (i.e. resemblance to the tubular branching of fungal hyphae) (Fig. 185.3).
Table 185.2 Dermoscopic structures observed in CMN
| Pattern | Definition | Characteristics |
| Reticular network | Honeycomb-like network of brown-black pigment | Quality Fine Thick Distribution Homogeneously throughout Patchy throughout Peripheral Specific type Linear fragments (hyphal-like) |
| Globules | Sharply circumscribed, round to oval aggregates of brown-black pigment | Quality Small Large Distribution Diffusely throughout (sparse or dense concentration) Central Specific type Cobblestone Target |
| Diffuse background pigmentation | Diffuse distribution of brown background pigment | Structureless Remnant structures Reticular networks fragments Few globules |
Fig. 185.2 This CMN has a reticular network pattern. It also has hypertrichosis and perifollicular hypopigmentation, both features commonly seen in CMN. A few central scattered globules can also be seen.
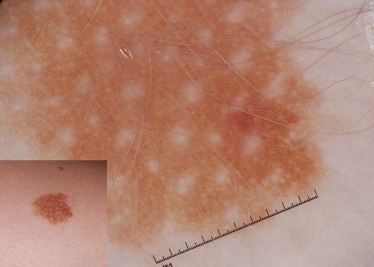
Fig. 185.3 This CMN has network fragments resembling hyphal elements. The lesion also has a few milia cysts, which are commonly seen in CMN.
A reticular network is the hallmark of melanocytic lesions and may also be seen in thin melanomas and benign acquired melanocytic naevi [6].
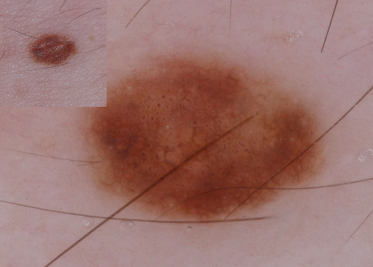
Globules consist of sharply circumscribed, round to oval aggregates that represent nests of melanin-containing naevus cells within the dermis (Fig. 185.4) [5,7]. Their colour can vary, based on their depth within the dermis, from light brown to dark brown to black to blue due to the Tyndall effect. Globules in CMN are characterized by their:
- quality, which can be small and/or large
- distribution, in which the globules can be present diffusely throughout the lesion in a sparse or dense distribution, or can cluster centrally with surrounding reticulation
- specific type, in which the globules form a cobblestone-like arrangement (Fig. 185.5) or target-like structures in which the globules appear to be surrounded by a halo (Fig. 185.6). Target-like structures include target network with globules and target globules. The target network consists of a globule centred in the ‘hole’ of the network, and corresponds to nests of melanocytes in the dermal papillae (see Fig. 185.6). The target globule consists of a darker globule centred within a larger and lighter coloured globule.
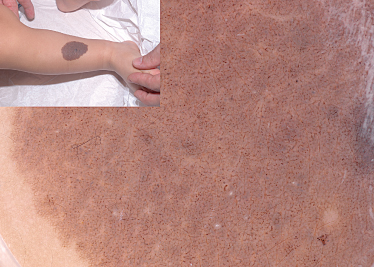
Fig. 185.4 CMN with brown and a few bluish globules throughout the entire lesion. This lesion also has hypertrichosis.
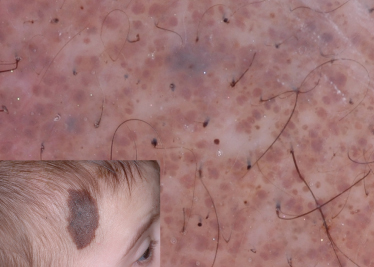
The diffuse background pigmentation is due to the diffuse distribution of brown background pigment, corresponding to presence of epidermal or dermal pigment (Fig. 185.7) [5]. Often these CMN are structureless, in that one cannot see any discernible dermoscopic structures, but some may have a few scattered reticular network fragments and/or globules.
Other dermoscopic structures frequently observed in CMN include (Table 185.3): milia-like cysts, hypertrichosis and perifollicular pigment changes. Milia-like cysts are white to yellow, rounded, often hazy structures that correlate histologically with intraepidermal keratin cysts and horn pseudocysts (see Fig. 185.3) [8]. They derive their name from their resemblance to the small seeds or millets of various grain grasses. Although milia-like cysts are one of the dermoscopic hallmarks of seborrhoeic keratosis, they may also be seen in papillomatous dermal naevi, CMN and, rarely, in melanomas. Hypertrichosis (see Figs 185.2, 185.4) is characteristic of CMN and is often accompanied by perifollicular hyperpigmentation or hypopigmentation (see Fig. 185.2). In addition, almost 70% of CMN will reveal vascular structures under dermoscopy [9]. The vascular structures most commonly encountered in CMN are comma vessels, dotted vessels, serpentine vessels and target network with vessels (see Table 185.3). The target network with vessels consists of a blood vessel centred in the ‘hole’ of the network, and corresponds to vessels in the dermal papillae.
Table 185.3 Other dermoscopic features seen in CMN
| Feature | Definition |
| Milia-like cysts | White to yellow, rounded, often hazy structures resembling the small seeds or millets of various grain grasses |
| Hypertrichosis | Increased number of thicker hairs |
| Perifollicular pigment changes | Hypopigmentation or hyperpigmentation occurring around the hair follicles |
| Vascular structures | Blood vessels of differing shapes including comma vessels, dotted vessels, and serpentine vessels can be seen in CMN. In addition, target network with vessels, consisting of a network in which the hole of the network contains a blood vessel, can also be seen in CMN |
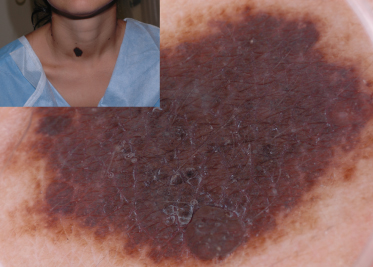
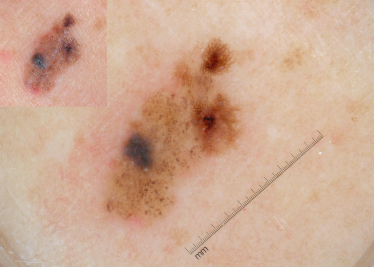
After identifying the local dermoscopic features commonly seen in CMN, it becomes apparent that these naevi often form specific dermoscopic patterns (Table 185.4). These global patterns are generally symmetrical and homogeneous. The five primary global patterns that emerge are (Fig. 185.8): reticular; globular; reticulo-globular (symmetrical); diffuse brown pigmentation; and multicomponent. Some CMNs are either primarily reticular (see Fig. 185.2) or primarily globular (see Figs 185.4, 185.5). Others exhibit peripheral reticulation in conjunction with central globules, and are designated reticulo-globular (Fig. 185.9). As noted earlier, CMN that primarily have diffuse brown pigmentation are often structureless but some may contain focal network fragments and/or a few scattered globules. CMN with a multicomponent pattern are difficult to differentiate from melanoma since they often have blotches, structureless areas, globules and reticulation distributed asymmetrically (Fig. 185.10).
Table 185.4 CMN dermoscopic patterns
| Global pattern | Definition |
| Reticular | Primarily reticular in pattern |
| Globular | Primarily globular in pattern |
| Reticulo-globular | Peripheral reticulation in conjunction with central globules (symmetrical) |
| Diffuse brown pigmentation | Primarily diffuse structureless pattern with or without reticular network fragments and/or remnant globules |
| Multicomponent | Symmetrically or asymmetrically distributed globules, reticulation, blotches, dots, veil, regression structures and/or structure less areas (3 or more of these need to be present) |
Fig. 185.8 A schematic of the most frequently encountered patterns in CMN and acquired naevi.
Reproduced from Handbook of Dermoscopy with permission from Informa.
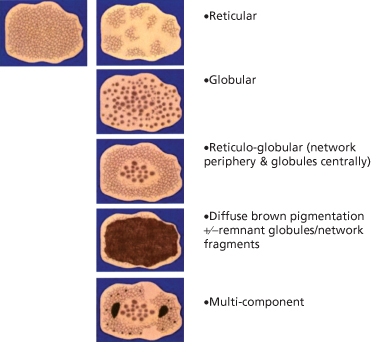
Fig. 185.10 This CMN has an asymmetrical multicomponent pattern with irregular blotches, globules and reticulation. Such lesions are difficult to differentiate from MM. This lesion also has milia cysts.
It has been observed that the type of pattern seen in a congenital naevus is dependent on the anatomical location of the naevus. The reticular pattern is observed most frequently on the extremities and the globular pattern is seen most frequently on the trunk, head and neck [9,10]. The difference in pattern based on body location may be explained by differences in melanoblast migration in the embryo. It has been theorized that CMN are a result of disruption in melanoblast migration and differentiation [9]. Migration of melanoblasts occurs in a cephalocaudal and proximal-distal sequence. Therefore, melanoblasts are found in the head/neck and dorsal trunk earlier in embryogenesis as compared to the extremities [11]. If there is a delay in the signalling responsible for directing the melanoblasts to the epidermis/dermoepidermal junction, the earlier migrating naevomelanoblasts may stall in the dermis, thereby producing a globular dermoscopic pattern. The later migrating naevomelanoblasts would be less affected by the delay in signalling and would probably reach a more superficial location at the dermoepidermal junction, producing a reticular pattern [9].
Although Unna’s theory of naevogenesis has long been used to explain the origin of junctional, compound and intradermal naevi, recent observations have brought into question Unna’s ‘Abtropfung-’ theory of naevogenesis [12]. The current opinion is that there are at least two pathways for naevogenesis. One, known as the congenital or endogenous pathway, is believed to be related to mutations in genes such as c-kit, c-met and NRAS. These mutations occur in utero, leading to melanoblast migration arrest in the dermis or epidermis. Based on location of migration arrest, rate of proliferation and timing of senescence, these melanocytic naevi can manifest a globular, reticular or reticulo-globular appearance (see Fig. 185.8). Those naevi in which no dermoscopic structures can be visualized are classified as having diffuse brown pigment (homogeneous) (see Fig. 185.8). Unlike acquired naevi, which frequently regress with age, once CMN undergo senescence they rarely regress and thus they are usually visible on the skin for the life of the individual [13]. The second conduit to naevogenesis is called the acquired or exogenous pathway and is discussed in the next section below.
References
1 Krengel S, Hauschild A, Schäfer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol 2006;155(1):1–8.
2 Marghoob AA, Borrego JP, Halpern AC. Congenital melanocytic nevi: treatment modalities and management options. Semin Cutan Med Surg 2003;22:21–32.
3 Braun RP, Calza AM, Krischer J, Saurat JH. The use of digital dermoscopy for the follow-up of congenital nevi: a pilot study. Pediatr Dermatol 2001;18(4):277–81.
4 Marghoob AA, Braun RP, Kopf AW. Atlas of Dermoscopy. London: Taylor and Francis, 2005.
5 Soyer HP, Smolle J, Pachernegg H, Kerl H. Surface microscopy: a new approach to the diagnosis of cutaneous pigmented tumors. Am J Dermatopathol 1989;11:1–10.
6 Kenet RO, Kang S, Kenet BJ, Fitzpatrick TB, Sober AJ, Barnhill RL. Clinical diagnosis of pigmented lesions using epiluminescence microscopy: grading protocol and atlas. Arch Dermatol 1993;129:157–74.
7 Soyer HP, Argenziano G, Chimenti S, Ruocco V. Dermoscopy of pigmented skin lesions. Eur J Dermatol 2001;11:270–7.
8 Massi D, de Giorgi V, Soyer HP. Histopathologic correlates of dermoscopic criteria. Dermatol Clin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree