Histopathological features of chronic GVHD show the epidermis to be atrophic with hyperkeratosis, thickening of the basement membrane and condensed/homogeneous connective tissue in the upper dermis. Basal layer vacuolar degeneration, inflammation and eosinophilic body formation are rare or absent. The dermis shows thickened, hyalinized collagen bundles, together with destruction of the adnexal structures.
In the setting of BMT, a skin biopsy is the preferred method of establishing a diagnosis of GVHD and in monitoring its course. Although GVHD can be recognized early in its course as an erythematous maculopapular rash, there is no one clinical or pathological feature that is specifically diagnostic of GVHD [31]. Individual keratinocyte cell necrosis may be induced by total body irradiation [32] and various cytotoxic drugs [33,34]. Evidence that cytotoxic agents can produce mild epidermal damage, including necrosis of occasional keratinocytes in association with a sparse lymphocytic infiltrate and some vacuolar alteration of basal epidermal cells, comes from studies of psoriatic patients treated with methotrexate and hydroxyurea [35,36]. Similar changes have been described with bleomycin, adriamycin and cyclophosphamide [34]. The author’s results showed 7 out of 17 pretransplant skin biopsies to be abnormal [37]. The specificity of the histological features of aGVHD was questioned by Sale and the Seattle team [34]. Some 49 skin biopsy specimens taken from marrow transplant patients, who received allogeneic, syngeneic or autologous marrow, were coded and studied ‘blindly’ by three pathologists. These authors concluded the following:
1 The early cutaneous histological changes do not permit a diagnosis of GVHD, except late in its evolution (after the 35th–40th day) when the effects of the cytotoxic agents have normally disappeared.
2 The presence of eosinophilic bodies, with or without satellite lymphocytes, is a necessary criterion, but is insufficient to confirm the diagnosis of GVHD as it can be caused by cytotoxic drugs. Their presence is, however, rare after the 19th day in patients who have received only cyclophosphamide and total body irradiation.
3 Certain situations require the repetition of skin biopsies at intervals of a few days. If epidermal lesions persist, the probability that they are due to cytotoxic agents decreases as the probability of GVHD increases. The histological diagnosis of GVHD therefore must take into account all other available relevant data. The author’s findings stress the importance of taking a pretransplant skin biopsy as a baseline.
Immunopathology
In a study by the author (J.H.) and co-workers, 14 skin biopsies of GVHD [38] were examined by an indirect immunoperoxidase technique using a panel of monoclonal antibodies. Controls included pretransplant skin biopsies, skin from normal healthy volunteers and skin from patients with lichen planus and cutaneous T-cell lymphoma.
The results demonstrated the following immunopathological features of cutaneous GVHD:
1 The lymphoid infiltrate of aGVHD is composed of mainly T-lymphocytes.
2 Helper (CD4+) T-lymphocytes, of donor origin, appear early and accumulate around blood vessels.
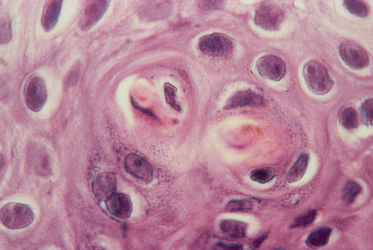
3 Suppressor/cytotoxic (CD8+) T-lymphocytes are found predominantly at the dermoepidermal junction and in the epidermis (Fig. 178.3).
4 There is a significant reduction in the number of detectable Langerhans cells in aGVHD.
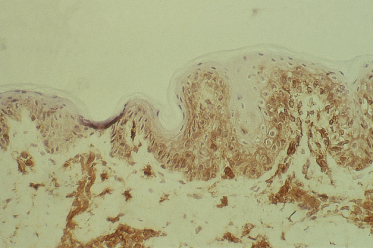
5 Acute GVHD is associated with HLA-DR expression of keratinocytes (Fig. 178.4).
6 There is a persistence of increased numbers of perivascular helper (CD4+) T-lymphocytes in chronic GVHD.
Fig. 178.3 CD8+ T-lymphocytes at the dermoepidermal junction and in the epidermis demonstrated using an indirect immunoperoxidase technique.
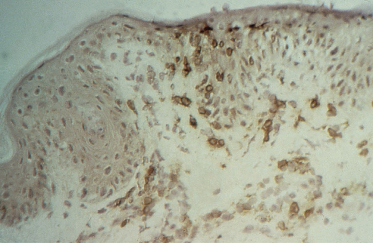
The presence of CD8+ cells in contact with destroyed keratinocytes strongly infers a cytopathic potential of these cells. Anti-Leu-7, which stains human NK cells as well as a subset of CD8+ cells, were few in number.
HLA-DR (Ia) staining of keratinocytes occurred in aGVHD, lichen planus and in two out of the six patients with cutaneous T-cell lymphoma. HLA-DR expression was not observed in normal or pretransplant skin biopsies. Studies in rats have shown that Ia staining can be induced in the skin during contact hypersensitivity reactions but is absent following mechanical or chemical damage to the skin [39]. Scheynius and Tjernlund [40] have demonstrated the induction of HLA-DR on keratinocytes during the tuberculin reaction. These facts suggest that HLA-DR or Ia staining by keratinocytes is a consequence of cellular immunity. In a murine model of acute cutaneous GVHD, Breathnach and Katz [41] have shown that the keratinocytes themselves synthesize Ia antigen in aGVHD.
The observation of a reduction in the number of Langerhans cells in aGVHD was first made by Lampert et al. [42] and Mason et al. [43] in F1 hybrid rats and subsequently in human GVHD [44]. However, in these studies there were no controls. The author’s studies (J.H.) confirmed that there is a significant decrease in the number of CD1+ dendritic (Langerhans) cells detectable in the skin biopsies of aGVHD, although there was a slight reduction in the number of Langerhans cells in pretransplant skin biopsies compared with the normal controls, presumably related to chemotherapy. These results suggest that the Langerhans cell is a primary target in cutaneous GVHD [37,38]. When Campath 1H (a monoclonal antibody directed against CD52) is used in vivo to T-cell deplete the graft, it may also reduce GVHD by removing Langerhans cells from the recipient.
Clinical Features.
Tissues that are primary targets include the epithelium of the skin, gastrointestinal tract and liver. The cutaneous signs are usually the earliest manifestation of GVHD [27,37,45]. It is traditional to divide the clinical manifestations into acute and chronic phases, occurring before and after day 100 respectively; this distinction is difficult to define precisely as acute lesions can transform and progress imperceptibly into chronic lesions, and a syndrome resembling aGVHD may develop well after day 100 and has particularly been described following the recently introduced very-low-intensity BMT procedures. New diagnostic criteria have therefore defined chronic GVHD based on actual physical manifestations rather than by the timing of its occurrence [46].
Out of the 100 BMT patients studied by the author (J.H.) [37], 76 developed GVHD (Table 178.2). Fever and skin rash occurred in all 76 patients (100%); 46 patients (61%) had acute gastrointestinal symptoms, and 28 patients (37%) had hepatic involvement. Out of the 76 patients, 23 (30%) developed chronic skin changes of GVHD.
Table 178.2 Incidence of graft-versus-host disease in patients after bone marrow transplant
Adapted from Harper 1985 [37].
| No. of patients | GVHD | |
| Aplastic anaemia | 16 | 12 |
| Leukaemias | ||
| ALL | 50 | 42 |
| AML | 5 | 3 |
| Fanconi anaemia | 3 | 3 |
| Immunodeficiency diseases | 11 | 4 |
| Inborn errors of metabolism | 15 | 12 |
| Total | 100 | 76 |
ALL, acute lymphocytic leukaemia; AML, acute myelocytic leukaemia.
Acute Disease
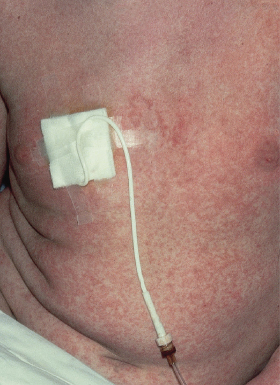
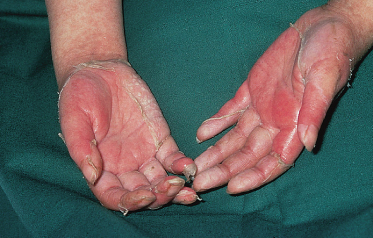
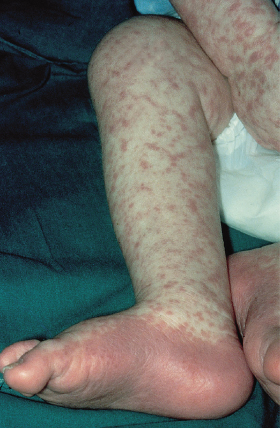
The most common presentation is a faint, erythematous maculopapular eruption on the trunk and limbs (Fig. 178.5), often starting on the face and affecting the palms (Fig. 178.6) and soles. Typically, aGVHD is seen at the time of haemopoietic reconstitution, 10–14 days post-transplant; in the author’s series this ranged from day 5 to day 60 post-graft. The more severe forms of aGVHD develop an erythroderma and subsequent epidermal separation, resulting in the appearance of bullae. The occurrence of TEN as a manifestation of fulminant aGVHD in humans was reported by Peck et al. [47] and was witnessed in 3 out of the author’s 100 patients. This has a high mortality: of the 100 patients, one died as a result of overwhelming aGVHD and the other two died of septicaemia. In areas of blister formation the separation is dermoepidermal, similar to that seen in drug-induced TEN. The severity of aGVHD is dependent on the degree of histoincompatibility and was inevitably worse when mismatched donors were used.
Other manifestations of aGVHD include fever and gastrointestinal and liver disturbance. Intestinal involvement is manifested by diarrhoea, nausea and vomiting. Abdominal pains and ileus are indicative of severe disease. Hepatic involvement causes an elevation in aspartate transaminase (AST) and alanine transaminase (ALT), hyperbilirubinaemia of the conjugated type and an elevation of alkaline phosphatase.
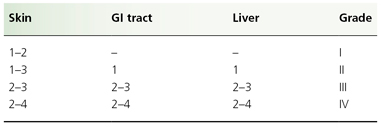
Individual organ system grading and calculation of an overall GVHD grade are shown in Tables 178.3 and 178.4, respectively. Patients with GVHD limited to grade I or II severity have a 6-month transplant-related mortality similar to patients with no GVHD. Patients with grade III GVHD, however, have a 50% risk of mortality at 6 months, whereas grade IV GVHD is usually fatal [48].
Table 178.3 Clinician’s grading of graft-versus-host disease: individual system
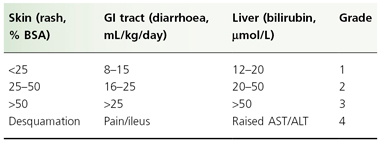
BSA, body surface area; ALT, alanine transaminase; AST, aspartate transaminase; GI, gastrointestinal.
Table 178.4 Clinician’s grading of graft-versus-host disease: overall grading
Although there remains a considerable degree of unpredictability in the occurrence of GVHD, there are many recognized risk factors. These include: HLA disparity between donor and recipient; minor MHC antigen differences, e.g. Y chromosome in male recipients of parous female marrow [49]; intensity of the pretransplant treatment [49]; increasing donor and recipient age; and viral infection after transplant [50]. Among recipients of umbilical cord blood (UCB) transplants there was no aGVHD of severity greater than grade I in recipients who were HLA matched or mismatched for one or two antigens [51]. Depending on all of these factors, the risk of GVHD can vary from 15% to 70% [52].
Chronic Disease
When patients survive aGVHD and other complications, especially infections, the cutaneous lesions either disappear completely or they gradually progress and evolve into the chronic manifestations of GVHD. The incidence of chronic cutaneous GVHD in the author’s patients was 30%. All of the patients who developed chronic skin changes had experienced previous acute manifestations. In a series of transplant patients studied by the Seattle group [53], chronic GVHD proved to be a significant problem in 19 out of 92 patients (21%) surviving 150 days or more; in five individuals, chronic GVHD apparently occurred without a preceding acute reaction. Chronic skin manifestations of GVHD include lichen planus-like lesions, pigmentary changes and sclerosis.
In the author’s series, a variety of lichenoid lesions occurred from day 29 to day 350 post-graft. Involvement of the buccal mucosa is a frequent finding. Saurat and Gluckman [54] stated that oral lesions always preceded the cutaneous lesions of chronic GVHD. However, this was not substantiated by this author’s observations. The mucosal lesions are similar to those seen in idiopathic lichen planus, with a white reticulate pattern affecting the buccal mucosa, gingiva, tongue and palate. Lichen planus lesions of the genitalia have also been reported [54,55] and were seen in one patient in the author’s series. The appearance of lichen planus papules on the skin shows similarities to that seen in idiopathic lichen planus [56–58], with polygonal, violaceous, shiny papules and Wickham’s striae. More often, however, the lesions are less typical of lichen planus although remaining lichenoid in nature. Lesions are often seen in a reticulate configuration, especially on the limbs (Fig. 178.7), suggesting some relationship with the underlying vascular network. The distribution, when widespread, does not tend to affect those areas of predilection seen in idiopathic lichen planus, such as the anterior aspects of the wrists.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree