As with amniocentesis, the main complication is pregnancy loss, which is about 2% [20]. There have been reports of an increased incidence of cutaneous haemangiomas in infants exposed to CVS antenatally. In one study, the reported incidence was 21%, which was three times higher than the incidence in the general population. It was found that the incidence of cutaneous haemangiomas, defined as strawberry birth marks, was significantly higher after CVS compared with second-trimester amniocentesis. This increased incidence was largely confined to patients who underwent a transcervical CVS procedure [21]. The mechanism by which haemangiomas develop in the general population is not understood and it would therefore be difficult to explain the above findings.
Other Antenatal Procedures
Other procedures that may leave scars or needle marks on the skin of the newborn include:
- fetal skin biopsies, which may be carried out for prenatal diagnosis of the severe genodermatoses. With the development of DNA-based prenatal diagnosis, this technique is now rarely required
- fetal liver biopsy, which is performed for the diagnosis of rare inborn errors of metabolism when other tests are not suitable, e.g. defects of the urea cycle enzymes
- fetal tumour biopsy, which can be used to confirm the diagnosis of teratomas or congenital adenomatoid malformations of the lung
- aspiration of fluid collections, such as fetal urine from a dilated bladder or a pleural effusion, which can be performed with ultrasound guidance
- therapeutic procedures, such as intrauterine shunting of obstructive uropathy, hydrocephalus or hydrothorax, and aspiration of fluid from renal cysts, ovarian cysts or ascites, all of which can be performed antenatally using ultrasound guidance.
Iatrogenic Injuries During Labour
Fetal Monitoring
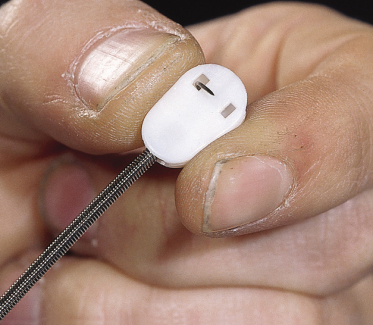
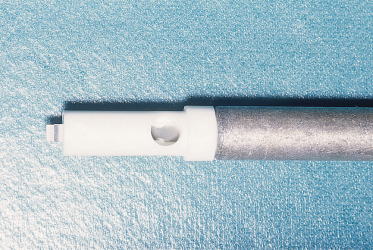
Continuous fetal heart rate monitoring is indicated during labour to monitor fetuses at risk of hypoxia [22]. This is achieved by using an ultrasonic transducer placed on the maternal abdominal wall or by using an electrode attached to the fetal scalp (Fig. 17.2). The latter method may produce bleeding at various levels in the scalp, including perforation of the periosteum and subperiosteal haemorrhage. Whenever fetal heart rate monitoring is being employed, it is vital that fetal acid–base status can be established from a scalp blood sample. A falling blood pH level indicates an impending problem and that action aimed at delivering the mother will probably be necessary. Scalp blood samples are obtained by performing a small incision in the scalp using the instrument shown in Figure 17.3. These injuries are a potential site of serious neonatal infection, which may be acquired during delivery. Persistent scalp bleeding due to fetal coagulopathy following fetal blood sampling has been reported [23]. Scalp gonococcal abscess following intrapartum fetal monitoring is another rare complication [24].
Injuries Acquired During Normal or Operative Deliveries
During labour, a caput succedaneum may develop. This is defined as soft tissue oedema and bruising of the presenting part. The swelling looks uncomfortable when it involves the breech and scrotum and very unpleasant in a face presentation. The bruising normally clears spontaneously in a few days. No specific treatment is needed but if there is extensive bruising, early phototherapy for hyperbilirubinaemia may be indicated.
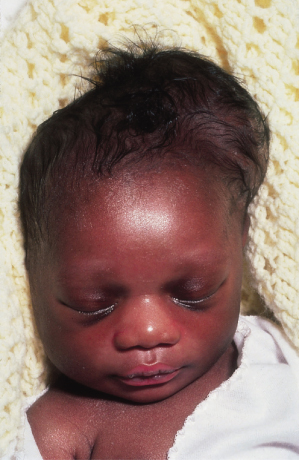
A cephalhaematoma (Fig. 17.4) is a subperiosteal haematoma that produces a lump over the scalp limited by the cranial sutures. Swelling is usually not visible until several hours after birth, since subperiosteal bleeding is a slow process. It is of little importance, although it may be many months before it finally disappears. The swelling may begin to calcify by the end of the second week and a few may remain for years as bony protrusions. No treatment is required, although phototherapy may be necessary for jaundice.
Rarely, a bleed may occur under the aponeurosis (subaponeurotic haemorrhage); the bleeding is not confined to a single cranial bone like a cephalhaematoma but spreads rapidly over the head and down towards the eyes. A significant fraction of the blood volume may be lost into this haematoma.
Bruising may be seen after manipulative deliveries and often in preterm infants. Vaginal breech deliveries also predispose to bleeding into the muscles of the legs and buttocks resulting from hypoxic capillary damage and increased hydrostatic pressure in the fetal dependent parts while awaiting delivery of the head [25].
Forceps Deliveries
In addition to the above, forceps deliveries may also cause erythema, abrasions, ecchymosis and subcutaneous fat necrosis of facial or scalp soft tissues. Their location depends on the area of application of the forceps. Moreover, in circumstances in which there is reduced area of contact, there is a predisposition to slipping of the forceps, which can result in visible facial and cranial markings [26]. Bruising of the skin after a forceps delivery may be so extensive as to produce anaemia or jaundice.
Vacuum Extractors
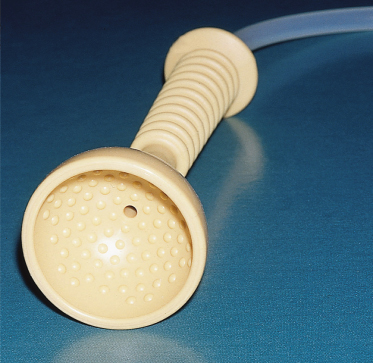
Delivery by the vacuum extractor (ventouse) is occasionally used instead of forceps when there is slow progress of labour. The vacuum is created using either a metal or a soft (plastic or silastic) cup applied onto the scalp and the baby is extracted by traction on the handle (Fig. 17.5). When air is evacuated from the cup, the scalp is sucked into the space inside the cup, so producing the chignon, which has a greater diameter above the rim than the rim itself (Fig. 17.6). Apart from producing jaundice, the chignon is a benign lesion in the majority of cases and it heals over a few days leaving no scars, but rarely it becomes lacerated and infected. Neonates delivered by vacuum have a higher incidence of cephalhaematomas [27].
Fig. 17.6 (a) Severe skin trauma and chignon following ventouse extraction. The ventouse is more usually applied over the vertex. (b) Later appearance of the lesion.
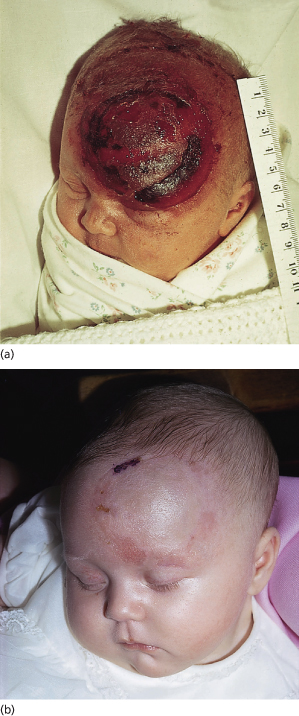
Abrasions to the scalp may also occur as a result of rotation or movement of the cap on the skin, caused by a badly placed cap. Sudden detachment of the cap can also cause skin damage. Ventouse extraction (vacuum extraction) can be used only for cephalic presentations and is contraindicated in preterm deliveries before 34 weeks’ gestation because of a significant incidence of complications [28]. Alopecia is a rare but important complication of ventouse deliveries [29].
Caesarean Section
Scalpel lacerations during caesarean section may be deep enough to require suturing and may occasionally become infected.
References
1 Jennings RW, Hunt TK. Overview of postnatal wound healing. In: Adzick NS, Longaker MT (eds) Fetal Wound Healing. New York: Elsevier, 1992: 25–52.
2 Galle PC, Meis PJ. Complications of amniocentesis: a review. J Reprod Med 1982;27:149–55.
3 Morrison WA , Hurley JV, Ahmad TS, Webster HR. Scar formation after skin injury to the human foetus in utero or the premature neonate. Br J Plastic 1999;52:6–11.
4 Rowlatt U. Intrauterine wound healing in a 20-week human fetus. Virchow’s Arch 1979;381:353–61.
5 Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg 2003;27:54–61.
6 Viljanto J, Thomasson B, Pikkarainen T et al. Fetal connective tissue regeneration. Acta Chir Scand 1975;141:85–9.
7 Adzick NS, Harrison MR, Glick PL et al. Comparison of fetal, newborn and adult wound healing by histologic, enzyme histochemical, and hydroxyproline determination. J Pediatr Surg 1985;20:315–19.
8 Hallock GG, Rice DC, McClure HM. In utero lip repair in the rhesus monkey: an update. Plast Reconstr Surg 1987;80:855–8.
9 Mulvihill SJ, Stone MM, Fonkalsrud EW. Trophic effect of amniotic fluid on fetal gastrointestinal development. J Surg Res 1986;40:291–6.
10 Dahl L, Hopwood JJ, Laurent BG et al. The concentration of hyaluronate in amniotic fluid. Biochem Med 1983;30:280–3.
11 Harris MC, Mennuti MT, Kline JA et al. Amniotic fluid fibronectin concentrations with advancing gestational age. Obstet Gynecol 1988;72:593–5.
12 Kratz G , Palmer B, Haegerstrand A. Effects of keratinocyte conditioned medium, amniotic fluid and EGF in reepithelialization of human skin wounds in vitro. Eur J Plastic Surg 1995;18:209–13.
13 Longaker MT, Adzick NS, Hall J et al. Studies in fetal wound healing. VII. Fetal wound healing may be modulated by elevated hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg 1990;25:430–3.
14 Adzick NS, Longaker MT. Characteristics of fetal tissue repair. In: Adzick NS, Longaker MT (eds) Fetal Wound Healing. New York: Elsevier, 1992: 53–70.
15 Ferguson MW. Wound healing-scar wars. Ulster Med J 1998 June;(Suppl 1):37–40.
16 Colwell AS, Logaker MT, Lorenz H. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound Repair Regen 2008;16(3):450–9.
17 Bevis DC. The antenatal prediction of haemolytic disease of the newborn. Lancet 1952;23:395–8.
18 Rodeck CH, Pandya PP. Prenatal diagnosis. In: Chamberlain G (ed) Turnbull’s Obstetrics. London: Churchill Livingstone, 2001: 169–96.
19 Epley SL, Hanson JW, Cruikshank DP. Fetal injury with midtrimester diagnostic amniocentesis. Obstet Gynecol 1979;53(1):77–80.
20 Mujezinovic F, Alferirevic Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol 2007;110(6):1425–6.
21 Burton KB, Schultz JC, Burd AB. An increased incidence of haemangiomas in infants born following chorionic villous sampling. Prenat Diagn 1995;15:209–14.
22 Whittle MJ, Martin WL. Fetal monitoring in labour. In: Chamberlain G (ed) Turnbull’s Obstetrics. London: Churchill Livingstone, 2001: 439–50.
23 Pachydakis A, Belgaumkar P, Sharmah A. Persistent scalp bleeding due to fetal coagulopathy following fetal blood sampling. Int J Gynaecol Obstet 2006;92(1):69–70.
24 Asnis DS, Brennessel DJ. Gonococcal scalp abscess: a risk of intrauterine monitoring. Clin Pediatr 1992;31(5):316–17.
25 Ralis ZA. Birth traumas to muscles in babies born by breech delivery and its possible fetal consequences. Arch Dis Child 1975;50:4–13.
26 Foster FMC. Robert Barnes and his obstetric forceps. Aust NZ J Obstet Gynecol 1971;11:139.
27 Johnson JH, Figueroa R, Garry D et al. Immediate maternal and neonatal effects of forceps and vacuum assisted deliveries. Obstet Gynecol 2004;103(3):513–18.
28 Chang AMZ, Lau TK. Operative vaginal delivery. In: Chamberlain G (ed) Turnbull’s Obstetrics. London: Churchill Livingstone, 2001: 581–99.
29 Aron R, Gordon W. Cicatricial alopecia: a complication of vaccum extraction. S Afr Med J 1972;19:671.
Iatrogenic Skin Disorders after Birth
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree