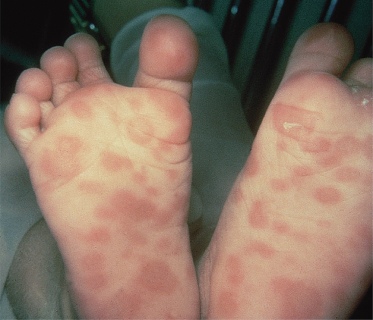
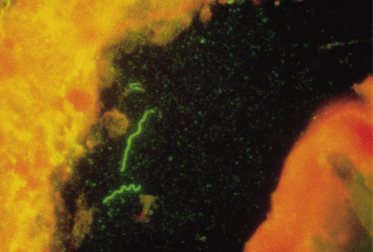
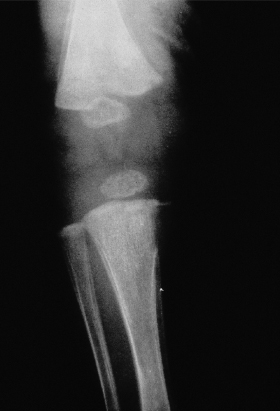
A positive cerebrospinal fluid (CSF) VDRL test in a newborn establishes a diagnosis of congenital syphilis but the overlap between CSF cell counts and protein determinations in infants with and without syphilis is so broad as to make the cell count and protein determination of no value [32]. Detection of T. pallidum may also be diagnostic [30,31] (Fig. 153.3). The bone lesions of congenital syphilis are those of a metaphysitis with either lucency or increased density seen in the long bones. The development of further involvement with erosion is a later finding. Erosion of the tibia is known as the cat bite or Wimberger sign. Periosteal involvement is also seen with congenital syphilis [31] (Fig. 153.4). Inflammation of bone associated with congenital syphilis can cause pain and impairment of movement. This is known as the pseudo-paralysis of Parrot. Occasionally, fractures are seen. Similar findings can be seen in child abuse. Diffuse bone involvement suggests congenital syphilis whilst an asymmetrical finding favours trauma [32]. A serological test for syphilis performed on the infant’s serum will be reactive in cases of congenital syphilis associated with significant bone pathology.
Late-onset congenital syphilis is manifest by evidence of continuing infection or evidence of stigmata. Most stigmata of congenital syphilis should be avoidable by adequate treatment but since an infant with late-onset congenital syphilis may never exhibit the early signs of congenital syphilis, and since 60% of cases of late-onset congenital syphilis were initially detected by serology alone, stigmata could theoretically develop because of treatment failure or lack of therapy. The findings of late-onset congenital syphilis as summarized by the American public health service include the following.
- Interstitial keratitis, a condition which leads to bilateral blindness and tends to develop around the time of puberty.
- Hutchinson’s teeth, a developmental abnormality of the upper and sometimes lower central incisors in which the teeth are notched and small, resulting in a gap between them.
- Mulberry molars in which the first molars show maldevelopment of the cusps and look like a mulberry.
- Eighth nerve deafness, which is infrequent and tends to develop around puberty.
- Neurosyphilis which has all the manifestations of neurosyphilis in acquired syphilis, including meningovascular, parenchymatous and gummatous neurosyphilis.
- Bone involvement which can be sclerotic (sabre shins, frontal bossing) or lytic (gummas resulting in destruction of the nasal bridge or the palate).
- Cutaneous involvement from healed syphilitic rhinitis (rhagades or cracks and fissures around the mouth).
- Cardiovascular lesions as seen in acquired lesions are reported but rare.
- Clutton’s joints, the painless hydrarthrosis of the knees.
Hutchinson’s triad consists of keratitis, dental abnormalities and deafness. Clutton’s joints, interstitital keratitis and deafness are not infectious and do not respond to penicillin.
Diagnosis.
Universal testing of women during pregnancy and at delivery will identify women with syphilis and those infants at risk for congenital syphilis [33–35]. It is more difficult to eliminate the possible diagnosis of congenital syphilis in an infant born to a mother with a reactive test for syphilis, especially if previous test information is not available. Results of serological tests based on endemic treponematoses are not distinguishable from syphilis, and may lead to misinterpretation (see Chapter 60). Endemic treponematoses never lead to congenital infections. Those antibodies are always passively acquired. Without a careful history, neither reactive maternal serological findings nor the height of the maternal titre can determine infectivity [35,36]. By following the American Centers for Disease Control (CDC) criteria for STDs, one will overtreat some children but will almost never miss possible cases [25]. These criteria were developed to identify the still symptom-free infected infant [33]. See Box 153.2 [25,39].
Box 153.2 Congenital Syphilis: Diagnostic Criteria According to Centers for Disease Control and Prevention Report on Sexually Transmitted Diseases (2002) and European STD Guidelines (2001)
Confirmed Congenital Infection
- Treponema pallidum demonstrated by dark-field examination microscopy or other specific staining of specimens for histopathological examination from skin lesions, lymph nodes, autopsy material or placenta
Presumed Congenital Infection
- A stillborn neonate with a positive treponemal test for syphilis
- Children with a positive treponemal test for syphilis in combination with persistent rhinitis, condylomata lata, osteitis, periostitis, osteochondritis, ascites, cutaneous and mucous membrane lesions, hepatitis, hepatosplenomegaly, glomerulonephritis, haemolytic anaemia
- Radiological abnormalities of the long bones suggestive of congenital syphilis
- A positive VDRL test in CSF
- A fourfold increase or more of the TPHA titre in the child opposed to the mother’s serum (obtained simultaneously at birth or at a later time)
- A fourfold increase or more of the titre of a cardiolipin/non-treponemal test in the child’s as opposed to the mother’s serum (obtained simultaneously at birth or at a later time)
- A positive 19S-IgM-FTA-abs test, EIA-IgM and/or IgM immunoblot for Treponema pallidum in the child’s serum
- A mother in whom syphilis was confirmed during pregnancy, but who was not adequately treated either before or during pregnancy
- A child >12 months of age with a positive treponemal serological test for syphilis (can also be acquired)
A diagnosis of infection with T. pallidum is made by either the detection of non-specific antibodies (non-treponemal antibodies) with confirmation by the detection of specific antibodies (treponemal antibodies) or the detection of T. pallidum. Non-treponemal antibodies are detected using the rapid plasma reagin card or the VDRL test. The tests are reactive, a quantitative titre obtained and the results are confirmed with a specific treponemal test. The specific treponemal tests are considered as confirmatory tests.
Current tests include the fluorescent treponemal antibody absorbed (FTA-ABS) test or the microhaemagglutination assay for antibody to T. pallidum (MHA-TP). In Europe, immunoglobulin G (IgG) and IgM enzyme immunoassays (EIAs) are also used for the diagnosis of syphilis. Non-standardized immunoblot assays are used by some investigators [30,31]. The IgM assays have the potential to detect cases of congenital syphilis as well as cases of acquired syphilis. The IgM test may not detect cases of congenital syphilis in which the patient has yet to develop symptoms [31].
Treponema pallidum is detected by dark-field examination, immunofluorescent antigen detection, the polymerase chain reaction (PCR) or the rabbit infectivity test. These tests approach 100% specificity but have variable sensitivity. They are of most value in the diagnosis of the early stages of congenital and acquired syphilis. The rabbit infectivity test is a useful standard against which to measure these other tests but it is only available in a research setting [30]. Infants suspected of highly probable disease should be thoroughly investigated. See Box 153.3 [25,39].
Box 153.3 Recommended Evaluation of Infants with Proven or Highly Probable Disease
- Direct dark-field examination or fluorescent antibody test or other specific test of skin lesions, lymph nodes or body fluids
- Serum quantitative non-treponemal and treponemal serological investigations. IgM serology is highly recommended
- Complete blood count and differential and platelet count
- CSF analysis for VDRL, cell count and protein
- Other tests as clinically indicated (long bone radiographs, chest radiograph, liver function tests, cranial ultrasound, ophthalmological examination)
Prognosis.
Both untreated congenital and acquired syphilis share some sequelae. Early and appropriate therapy results in a good outcome [34]. Whilst there is not a large enough experience with late congenital syphilis to evaluate the effectiveness of penicillin therapy, case reports have not shown penicillin to be helpful [37]. Failures from appropriate therapy of congenital syphilis are not reported but there have been short-term failures of benzathine penicillin therapy. All patients have been retreated appropriately [38]. Careful follow-up of all infants at risk of congenital syphilis is essential to ensure that the treatment given was effective [36].
Differential Diagnosis.
Syphilis is the great imitator. When acquired syphilis in children presents with a primary chancre, the differential diagnosis should be considered for a suspected bacterial skin infection which does not respond to treatment. The differential diagnosis also includes HSV and chancroid. The rash of secondary syphilis can be confused with any of the papulosquamous disorders, with the greatest likelihood of confusion with pityriasis rosea. The systemic manifestations of acquired syphilis are non-specific except for such findings as epitrochlear adenopathy.
Both congenital or acquired syphilis can present with a cerebrospinal pleocytosis. There are very few findings in the CSF that would specifically indicate syphilis other than tabes dorsalis.
The bullous skin findings of congenital syphilis can be seen with epidermolysis bullosa, dermatitis herpetiformis staphylococcal infection or mastocytosis [40]. Hepatosplenomegaly can be seen in any of the congenital infections such as toxoplasmosis, rubella or cytomegalovirus. The anaemia of congenital syphilis can be seen in any other cause of hydrops fetalis, especially parvovirus infection. The bone lesions of congenital syphilis can be confused with either infection or child abuse.
Treatment.
Penicillin G, administered parenterally, is the preferred drug for treatment of all stages of syphilis [25]. First-choice treatment exists of benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units in a single dose. Patients who also have symptoms or signs suggesting neurological or ophthalmic disease should have an evaluation including CSF analysis and ocular slit-lamp examination. All patients should also be tested for HIV infection [25]. Administration of IM benzathine penicillin is painful; dilution of the penicilline with 1% lidocaine HCL may reduce pain symptoms [41].
Treatment of congenital syphilis involves:
- adequate treatment of the mother before pregnancy
- adequate treatment of the mother during pregnancy, preferably in the first half of pregnancy but definitely before the last month of pregnancy, or
- adequate treatment of the infant either at delivery or postnatally when symptoms develop.
Any strategy involving maternal therapy must involve therapy of all sexual partners or the treated mother will become reinfected. Adequate maternal therapy is defined as either one injection of benzathine penicillin (2.4 million units) for early syphilis (primary and secondary syphilis) or one injection per week for 3 weeks of benzathine penicillin to a total dose of 7.2 million units. As a result of this therapy, patients with early syphilis should show a fourfold decrease in non-treponemal titre or return to become negative. Patients with late syphilis should have stable or declining titres of less than or equal to 1 : 4 [25].
Most pregnant women with reactive syphilis serologies do not fit into any of these categories, frequently because an appropriate fall in titre is not documentable before delivery. Thus, many infants at risk for congenital syphilis are treated for congenital syphilis because adequate therapy in their mother cannot be established with certainty.
The therapy of congenital syphilis is 10–14 days of penicillin G (50,000/kg/dose every 12 h for the first week of life and every 8 h thereafter). Although therapy with intravenous penicillin G is one option, the authors’ experience with procaine penicillin 50,000 units/kg given intramuscularly daily for 10 days has been good. There have been no reported treatment failures with either penicillin G or procaine penicillin while therapy with benzathine penicillin as a single injection has resulted in some treatment failures [38,42]. The cost–benefit analysis of treatment with benzathine penicillin with discharge thereafter versus 10 days of parenteral therapy has yet to be determined [43].
As in acquired syphilis, an appropriate fall in the non-treponemal serology is expected. Infants who are treated late in the course of their disease may never become seronegative [44]. Fifteen percent of patients with early syphilis treated with the recommended therapy will not achieve a twofold dilution decline in non-treponemal titre [25]. Non-penicillin therapies of congenital syphilis have not been evaluated and should not be used. Appropriate doses of ampicillin can be considered as equivalent to penicillin [25]. One should bear in mind that treatment failures can occur.
Box 153.4 Recommended Treatment Regimens for Children with Acquired or Congenital Syphilis
Infants With Congenital Syphilis
- Aqueous crystalline penicillin G 150,000 units/kg/day, administered as 50,000 units/kg/dose IV every 8 hours during 10 days
Or
- Procaine penicillin G 50,000 units/kg/dose IM in a single dose during 10 days
Older Children
- Aqueous crystalline penicillin G 200,000–300,000 units/kg/day IV, administered as 50,000 units/kg every 6 hours for 10 days
- No proven alternatives to penicillin are available. Erythromycin (in children) and doxycycline or tetracycline (only in adults) are claimed to be effective, but these drugs are not mentioned in the CDC STD guidelines
References
1 Dennie CC. A History of Syphilis. Springfield, Illinois: C.C. Thomas, 1962.
2 Carpenter G. The Syphilis of Children in Every-Day Practice. New York: William Wood, 1901.
3 Harter CA, Benirschke K. Fetal syphilis in the first trimester. Obstet Gynecol 1976;124:705–11.
4 Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol 1973;107:373–7.
5 US Public Health Service. Syphilis: A Synopsis. Washington, DC: US Public Health Service, 1968.
6 Schulz KP et al. In: Holmes et al. (eds) Sexually Transmitted Diseases. New York: McGraw-Hill, 1990.
7 Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis 1980;7:161–4.
8 Fiumara N. Treatment of secondary syphilis: an evaluation of 204 patients. Sex Transm Dis 1977;4:96–9.
9 Echols SK, Shupp DL, Schroeter AL. Acquired secondary syphilis in a child. J Am Acad Dermatol 1990;22(2 Pt 1):313–14.
10 Horowitz S, Chadwick DL. Syphilis as a sole indicator of sexual abuse: two cases with no intervention. Child Abuse Negl 1990;14:129–32.
11 Waugh JR. Acquired syphilis of infancy and childhood. Am J Syph Gon Ven Dis 1938;22:607–22.
12 Schoch AG, Long WE. Acquired syphilis in children. Am J Syph Gon Ven Dis 1939;23:186–7.
13 Smith FR. Acquired syphilis in children. Am J Gon Ven Dis 1938;23:165–85.
14 Ackerman AB, Goldfaden G, Cosmides JC. Acquired syphilis in early childhood. Arch Dermatol 1972;106:92–3.
15 Ginsburg CM. Acquired syphilis in prepubertal children. Pediatr Infect Dis 1983;2:232–4.
16 Aloi F. Lip syphilitic chancre in a child (letter). Pediatr Dermatol 1987;4:63.
17 Goldenring JM. Secondary syphilis in a prepubertal child. Differentiating condylomata lata from condylomata acuminata. NY State J Med 1989;89:180–1.
18 Tomeh MO, Wilfert CM. Venereal diseases of infants and children at Duke University Medical Center. N C Med J 1973;34:109–13.
19 Schwarcz SK, Whittington WL. Sexual assault and sexually transmitted diseases: detection and management in adults and children. Rev Infect Dis 1990;12(Suppl 6):S682–90.
20 Rimsza ME, Niggemann EH. Medical evaluation of sexually abused children: a review of 311 cases. Pediatrics 1982;69:9–14.
21 De Jong AR. Sexually transmitted diseases in sexually abused children. Sex Transm Dis 1986;13:123–6.
22 White ST, Loda FA, Ingram DL et al. Sexually transmitted diseases in sexually abused children. Pediatrics 1983;72:16–21.
23 Rawstron SA, Bromberg K. Comparison of maternal and newborn serologic tests for syphilis. Am J Dis Child 1991;145:1383–8.
24 Dorfman DR, Claser JH. Congenital syphilis presenting in infants after the newborn period. N Engl J Med 1990;323:1299–302.
25 Centers for Disease Control. 2002 sexually transmitted diseases treatment guidelines. MMWR 2002;51:1–78.
26 Ingraham NR. The diagnosis of infantile congenital syphilis during the period of doubt. Am J Syph Neurol 1935;19:547–80.
27 Cremin BJ, Fisher RM. The lesions of congenital syphilis. Br J Radiol 1970;43:333–41.
28 Rawstron SA, Jenkins S, Blanchard S, Li PW, Bromberg K. Maternal and congenital syphilis in Brooklyn, NY. Epidemiology, transmission, and diagnosis. Am J Dis Child 1983;147:727–31.
29 Shah MC, Barton LL. Congenital syphilitic hepatitis. Pediatr Infect Dis J 1989;8:891–2.
30 Sanchez PJ, Wendel GD Jr, Grimprel E et al. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J Infect Dis 1993;167:148–57.
31 Bromberg K, Rawstron S, Tannis G. Diagnosis of congenital syphilis by combining Treponema pallidum-specific IgM detection with immunofluorescent antigen detection for T. pallidum. J Infect Dis 1993;168:238–42.
32 Fiser RH, Kaplan J, Holder JC. Congenital syphilis mimicking the battered child syndrome. How does one tell them apart? Clin Pediatr (Phila) 1972;11:305–7.
33 Boot JM, Oranje AP, de Groot R et al. Congenital syphilis. Int J STD AIDS 1992;3:161–7.
34 Boot JM, Menke HE, van Eijk RVW et al. Congenital syphilis in The Netherlands: cause and parental characteristics. Genito Urin Med 1988;64:298–302.
35 Boot JM, Oranje AP, Menke HE et al. Congenital syphilis in The Netherlands: diagnosis and clinical features. Genito Urin Med 1989;65:300–3.
36 Glaser JH. Centers for Disease Control prevention guidelines for congenital syphilis. J Pediatr 1996;129:488–90.
37 Wiggelinkhuizen J, Mason R. Congenital neurosyphilis and juvenile paresis: a forgotten entity? Clin Pediatr 1980;19:142.
38 Beck-Sague C, Alexander ER. Failure of benzathine penicillin G treatment in early congenital syphilis. Pediatr Infect Dis 1987;6:1061–4.
39 Goh BT, van Voorst Vader PC. European guideline for the management of syphilis. Int J STD & AIDS 2001;12:14–26.
40 Oranje AP, Soekanto W, Sukardi A et al. Diffuse cutaneous mastocytosis mimicking staphylococcal scalded-skin syndrome: report of three cases. Pediatr Dermatol 1991;8:147–51.
41 Amir J, Ginat S, Cohen YH, Marcus TE. Lidocaine as a diluent for administration of benzathine penicillin. Pediatr Infect Dis J 1998;17(10):890–3.
42 Hardy JB, Hardy PH, Oppenheimer EH, Ryan SJ Jr, Sheff RN. Failure of penicillin in a newborn with congenital syphilis. JAMA 1970;212:1345–9.
43 De Lissovoy G, Zenilman J, Nelson KE, Ahmed F, Celentano DD. The cost of a preventable disease: estimated US national medical expenditures for congenital syphilis, 1990. Publ Health Rep 1995;110:403–9.
44 Smith FRJ. Congenital syphilis in children, results of treatment, 521 patients. Part I. Am J Syph Neurol 1935;532–46.
Gonorrhoea
Definition.
Neisseria gonorrhoeae (gonococci) are non-motile, non-spore-forming Gram-negative diplococci (they grow in pairs). Gonococcal infection in children is acquired either perinatally, from an infected mother to a newborn, or by intimate contact (almost always sexual) in older children.
History.
Gonorrhoea is one of the oldest known human illnesses. While references to urethral discharge are made in the Old Testament, in the fourth and fifth centuries BC Hippocrates wrote of gonorrhoea, although the term gonorrhoea (‘flow of seed/semen’) was not introduced until the second century by Galen. Neisser, who also discovered that the agent could be found in cases of ophthalmia neonatorum, finally identified the causal organism of gonorrhoea in 1879. Leistikow and Loeffler in 1882 were the first to culture the organism, and around the same time in 1881 Credé, who had been working on neonatal ophthalmia, started to use silver nitrate instillation into the eyes of newborns to prevent gonococcal ophthalmia, a common cause of blindness. The use of silver nitrate prophylaxis reduced the incidence of neonatal gonococcal ophthalmia from more than 10% to 0.5% [1].
Epidemic vulvovaginitis in girls was a common disease in the early 20th century before the advent of penicillin therapy, and was believed to be extremely contagious, requiring only superficial contact for transmission [2]. However, careful study of infected girls in controlled circumstances showed that gonococcal vulvovaginitis was not contagious (no transmission was seen from infected to non-infected girls on a ward, although there was no effective treatment at that time) [2]. The conclusion that ‘transmission of the disease requires intimate contact between an infected adult or child and non-infected child’ remains today.
Aetiology and Pathogenesis.
Gonorrhoea is an STD which can only be acquired by intimate contact, almost always sexual [3]. Humans are the only natural host and direct mucous membrane contact is necessary to spread disease [4]. Studies of STDs in various populations of children being evaluated for sexual abuse have shown rates varying from 2.8% [5], 4.7% [6], 7.4% [7] to 18.2% and 36.8% [8], the range being a function of the prevalence of N. gonorrhoeae in the community. In addition, a heightened suspicion for sexual abuse in recent times has resulted in an apparent decreased prevalence due to more evaluations in asymptomatic children.
In the neonatal period, infection is acquired perinatally from the mother by passage through an infected birth canal. In older prepubertal children, the infection is almost always sexually acquired, usually by sexual abuse from an adult, occasionally by sexual play between children [9], although even in these cases there is often abuse or exploitation of younger children by older ones who introduce the infection [10]. In older postpubertal children, consensual sexual activity is the usual source of infection, although the sexual activity can still be associated with abuse [11]. The role of fomites in the spread of disease is not clear, but is probably extremely uncommon. The only well-documented spread of gonococcal infection in a non-sexual manner was a hospital outbreak of neonatal gonococcal infection probably spread by contaminated rectal thermometers [12]. Careful interviewing enabled a history of sexual contact to be elicited in 44 of 45 1–9 year olds with gonorrhoea [13]. Similarly, a history of sexual contact was obtained in 90–100% [5,14] of children 5–12 years of age with gonorrhoea, and 35–75% [5,14] of children 1–5 years of age.
If gonorrhoea is highly associated with sexual contact in verbal children, it follows that this is the most likely mode of transmission in non-verbal children. Repeated interviews by sympathetic and skilled workers may be necessary to elicit a history of abuse [15]. Sometimes the history of abuse may not be revealed until years later [16].
Pathology.
Gonococcal infections start with the organism adhering to the mucosal cells which is mediated by pili and other surface proteins. Stratified squamous cells can resist invasion, but columnar epithelium is susceptible. This explains the distribution of infection: urethra, Skene and Bartholin glands, cervix and fallopian tubes in females; urethra, prostate, seminal vesicles and epididymis in males; and rectum, pharynx and conjunctivae in both sexes. Prepubertal girls are susceptible to vaginal infections with N. gonorrhoeae because of the alkaline pH and lack of oestrogenization, whereas postpubertal girls develop cervical but not vaginal infections. The organism is engulfed by endocytosis of the cell into vacuoles, where they may replicate and eventually exit from the basal surface of the epithelial cell to the subepithelial tissues [17]. There is a marked inflammatory response at the site of inoculation with a polymorphonuclear leucocyte response, purulent material being exuded from the surface and submucosal microabscess formation.
The pathology of the skin lesions in disseminated gonococcal infection (DGI) consists of haemorrhage, vasculitis and a moderately heavy inflammatory cell presence, mostly polymorphonuclear leucocytes but a variable presence of mononuclear cells [18]. Thrombosis of the small venules and arterioles of the dermis is common. Epidermal changes range from minimal oedema with few polymorphonuclear cells and red blood cells to intradermal vesicles or pustules. The organisms are only detected in the skin lesions by Gram stain or culture in about 10% of cases [19]. However, the presence of organisms can be detected in about 57% of skin lesions with the use of immunofluorescent stains [19].
Clinical Features
Infection in Infants
In newborns, the disease is acquired perinatally from an infected mother during delivery through an infected birth canal with direct mucosal contact from infected cervical secretions of the mother to mucous membranes (conjunctiva, pharynx) of the baby. Without prophylaxis, neonatal gonococcal conjunctivitis occurred in 42% of babies born to mothers with gonorrhoea, with 7% also having orogastric contamination with N. gonorrhoeae [20]. The prevalence of maternal disease varies depending on the prevalence of gonorrhoea in the community at any particular time. The rate of maternal gonorrhoea in most American populations is less than 5%, though rates in Africa are higher (5–10% or more). Prenatal care with screening and treatment is effective at preventing neonatal infections in high-risk populations. In addition, neonatal ocular prophylaxis can reduce the incidence of gonococcal ophthalmia in newborns with infected mothers by 83–93% [21]. However, universal screening of all pregnant women and neonatal ocular prophylaxis are not cost-effective when maternal gonococcal infections are infrequent (<1%), as is found in many industrialized countries. In the USA, both universal maternal screening and neonatal ocular prophylaxis are used to decrease the likelihood of neonatal infection, with neonatal ocular prophylaxis required by law in most states.
Conjunctivitis is the most common manifestation in newborns [22]. The conjunctivitis usually presents at 2–5 days of life (range 0–28 days), initially as a watery conjunctival exudate which rapidly becomes purulent and thick and may be blood-tinged. The conjunctiva and eyelid are oedematous and if the infection goes untreated, keratitis, iridocyclitis, corneal ulceration and perforation can ensue with blindness as a consequence. Other manifestations seen at this age are other local infections such as scalp abscesses (associated with scalp electrodes) or systemic infections caused by gonococcaemia and subsequent seeding of organisms to other areas, for example sepsis, arthritis, meningitis and pneumonia.
Gonococcal infections may also be asymptomatic, with cultures positive from the oropharynx, vagina and rectum. The most common manifestation of systemic infections in neonates is arthritis. This presents between 1 and 4 weeks after delivery. In the largest series [12], the neonates were often irritable and febrile, but neonates may also present with joint swelling alone, with no systemic findings [23]. Some had skin lesions (not described) and superficial abscesses before they developed arthritis. The arthritis was usually polyarticular, with wrists, knee and shoulder joints most commonly affected.
Older Children
Gonococcal infections in older children are usually local infections (vaginitis, urethritis, conjunctivitis). Disseminated infections are uncommon but do occur in preadolescent children, with arthritis and DGI being the most common manifestations. However, many gonococcal infections are asymptomatic, with 15–44% of genital infections in children being asymptomatic [5,6].
The most common manifestation is vulvovaginitis. This usually presents as a profuse purulent vaginal discharge ranging from white, cream, yellow or green in colour which stains the underwear. However, the vaginal discharge may be minimal and confused with a benign discharge [24]. Associated pruritus, vulval erythema and dysuria may also be present [25]. Rarely, prepubertal girls may have lower abdominal pain and fever in association with gonococcal vaginitis, suggesting ascending pelvic infection [26]. Symptoms are usually present for less than a week (median 3 days), but some children have symptoms for more than 2 weeks or even months before they are brought for evaluation. Gonococcal infections are less frequent in boys and the usual presentation is a urethral discharge associated with urethritis. The discharge may be copious or scant, and rarely may be associated with penile oedema [27] or the testicular swelling of epididymitis [25]. Dysuria may also be present. Gonococcal conjunctivitis can also present outside the neonatal period, usually in association with autoinoculation from a genital infection in the same patient. Conjunctivitis is often severe with profuse purulent discharge, chemosis, eyelid oedema and ulcerative keratitis, and presentations may mimic orbital cellulitis. On rare occasions, the conjunctivitis is the only gonococcal infection present, and the source of the infection is obviously from another person, often in the family [28]. The method of transmission in these cases is not clear, but non-sexual transmission is unlikely.
Pharyngeal and rectal infections are fairly common, but prevalence varies in different populations [25], probably reflecting sexual practices. Pharyngeal infections are seen in 15–54% of children with gonococcal infections [25,29]. Almost all pharyngeal and rectal infections are asymptomatic, and are detected by routinely screening these sites in children who are suspected to have been sexually abused, or who have genital discharges [30]. Rarely, pharyngeal infections are symptomatic [31]. Rectal infections are common in girls, probably due to the proximity of the vagina and anus with the possibility of contamination of the anus with vaginal discharge. Rectal cultures may be positive in up to 50% of girls with positive vaginal gonococcal cultures [25]. Most rectal infections in girls are asymptomatic, but occasionally there are symptoms [32], usually a purulent rectal discharge with rectal pain, blood or mucus in the stools and perianal itching or burning. Symptomatic rectal infections are associated with penile-rectal penetration. Rectal infections are rarely seen in boys, and are associated with anal intercourse.
Infection in adolescents is very similar to that seen in adults. In girls, the presentation is with cervicitis, DGI, perihepatitis (Fitz–Hugh–Curtis syndrome), salpingitis and occasionally proctitis. In boys, urethritis, epididymitis and occasionally proctitis are the usual presentations. The most serious potential complication of gonococcal infection in adolescent girls is salpingitis and pelvic inflammatory disease (PID), which is seen in about 15% of adolescent girls with gonococcal infections. DGI can be seen in the adolescent population, although it is more common in adults, with ages 15–35 years having the highest risk for DGI. DGI is more common in women than men, and more common during the first few days of menstruation and during pregnancy. DGI can result from a primary infection at any site including the cervix, urethra, anal canal, pharynx and conjunctiva. The presentation of DGI is usually with dermatitis, arthropathy or both. Skin involvement is seen in about 50–70% of patients with DGI [33]. The skin lesions are usually multiple, erythematous, maculopapular, vesicular, haemorrhagic, pustular or necrotic lesions. They often progress from papules to pustular, haemorrhagic or necrotic lesions, and the presence of lesions in different stages of evolution is typical of DGI [33]. The lesions are usually on the extremities and number from one to 40, and range in size from 1 to 20 mm [34].
Joint symptoms are seen at the initial presentation of DGI in more than 90% of patients [33]. The most commonly involved joints are the knees, ankles, wrists, elbows and the small joints of the hands and feet. Polyarthralgia is common and may be migratory. The symptoms range from mild to severe and include arthralgias with no inflammation to arthritis with synovial effusion and even joint destruction. Tenosynovitis is frequent [35]. It has been hypothesized that DGI consists of an early bacteraemic stage which if left untreated leads to a septic joint stage [33,35], though not all patients fit this picture. Some bacteriological findings are consistent with this hypothesis; for example, blood cultures are often positive in the early phase and joint fluid may be culture positive in the later stage. Positive blood and synovial fluid cultures are almost always mutually exclusive [35].
Diagnosis.
Since the diagnosis of gonococcal infections in children has serious medicolegal implications, it is essential to use only standard culture systems for diagnostic purposes in children [36]. Non-culture gonococcal tests such as Gram-stained smears, EIA tests and DNA probes should not be used for diagnostic purposes in children. Although Gram-stained smears of specimens can be useful in clinical practice, and are recommended for screening, they are inadequate for definitive diagnostic purposes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree