Cutaneous vessels are prominent, reflecting the thinness of the skin and the level of prematurity. Natal teeth have occasionally been described. A shedding of hyperkeratotic skin or epithelium may be seen and, rarely, the presence of denuded skin has been reported. A number of non-cutaneous features may also be present.
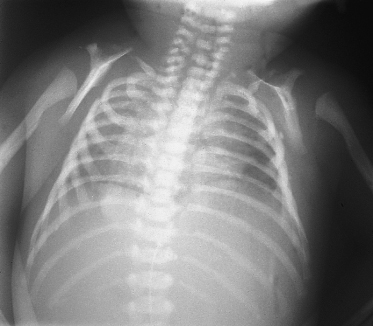
Skeletal abnormalities are frequently described: osteodysplasia of the clavicles (Fig. 15.2) and skull, and modelling defects of long bones are the most common that have been reported [1,6,7,9,11]. The placenta is frequently enlarged at birth and may show signs of chorio-angiosis [15]. Occasional associations reported include choanal atresia, hypospadias, microcephaly, adrenal hyoplasia, thymus hypoplasia, dextrocardia, atrial septal defect, transposition of the great arteries and microcolon but these may be unrelated.
Fig. 15.2 Chest X-ray showing osteodysplasia of the clavicles and modelling defects of the proximal humeri.
Prenatally, decreased fetal movements may be noted by the mother and polyhydramnios may develop.
Prognosis.
All cases to date have died within hours or within a few weeks, depending on the degree of active respiratory intervention. The cause of death is respiratory failure as the chest is unable to expand within the rigid cuirass (suit of armour) of the skin. One case survived for 4 months but the arrest in cutaneous maturation of this fetus appears to have occurred somewhat later than in the other cases [29].
Differential Diagnosis.
The clinical, radiological and histological features of restrictive dermopathy are very distinctive and should not be readily confused with other neonatal stiff skin syndromes (infantile systemic hyalinosis, Winchester syndrome and congenital fascial dystrophy) [34–37], sclerema neonatorum [38] or other rare fetal akinesia syndromes [39–41]. Fluorescent labelling of prelamin A in fetal blood leucocytes may provide a quick and easy test to diagnose loss of ZMPSTE24 function [24] but may not be reliable as a prenatal diagnostic test.
Treatment.
Initial treatment consists of hydration, nursing in a warm, humidifed environment, analgesia and respiratory support. Once the diagnosis is confirmed and the implications discussed with the parents, active treatment is usually withdrawn. Genetic counselling should be offered to the affected families.
Recent in vitro studies [42–44] have shown that farnesyltransferase inhibitors (FTI) can decrease the accumulation of ‘mutated’ farnesylated prelamin A at the nuclear envelope and reduce the mis-shapen nuclei. In vivo administration of FTI to a mouse model of progeria improved the clinical phenotype but it is not known if this approach could help restrictive dermopathy (where clearly any therapy would need to be given in utero in view of the early lethality) but development of a mouse model should help in further studies.
Prenatal Diagnosis.
Prenatal ultrasound and late prenatal skin biopsy (week 20) are unreliable [45–48]. Mutation analysis of the LMNA and ZMPSTE24 genes now allows accurate prenatal diagnosis [25,26].
References
1 Witt DR, Hayden MR, Holbrook KA et al. Restrictive dermopathy: a newly recognized autosomal recessive skin dysplasia. Am J Med Genet 1986;24:631–48.
2 Leschot NJ, Treffers PE, Becker-Bloemkolk MJ et al. Severe congenital skin defects in a newborn. Eur J Obstet Gynecol Reprod Biol 1980;10:381–8.
3 Carmi R, Sofer S, Karplus M et al. Aplasia cutis congenita in two sibs discordant for pyloric atresia. Am J Med Genet 1982;11:319–28.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








