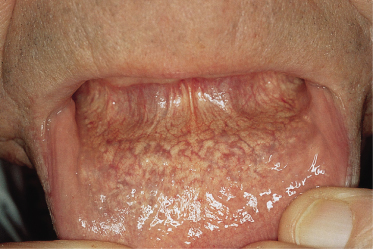
Fig. 144.2 Van Gieson stain, showing fragmentation of elastic fibres in PXE (a) and in normal elastic fibres in the skin of an unaffected individual for comparison (b).
On staining with Alcian blue, there is a marked increase in mucopolysaccharides. Upon digestion with hyaluronidase, it can be shown that much of this Alcian blue-positive material is hyaluronic acid. A number of studies have demonstrated marked increases in dermal mucopolysaccharides in lesional skin of patients with PXE. Both dermatan sulphate and hyaluronic acid are increased [46–48]. Of great interest, the increase in mucopolysaccharides is also demonstrable in clinically unaffected skin of patients with PXE. This has been clearly shown in non-lesional buttock skin. The authors suggested that deposition of mucopolysaccharides precedes the calcification of elastic tissue, and the negatively charged mucopolysaccharides may contribute to elastic tissue calcification by electrostatic forces [46]. This leads to the intriguing possibility that by preventing this increase in mucopolysaccharides, we may one day be able to prevent the calcification that occurs in PXE.
On electron microscopy, profound changes in both elastic tissue and collagen have been described. Elastic fibres contain irregularly shaped holes and electron-dense bodies. Some elastic fibres contain holes surrounded by a striking discrete electron-dense borderline [13,49].
Numerous abnormalities of collagen have also been described, including collagen fibrils that are thin, thick, laterally fused and twisted as well as decreased in number and broken down to particles [13,49–53].
Clinical Features.
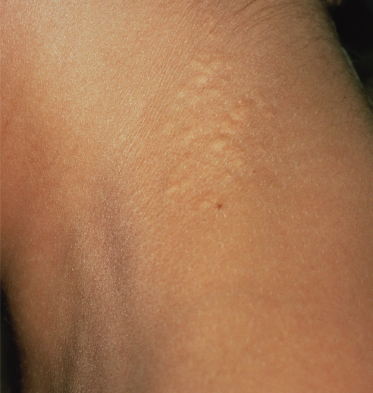
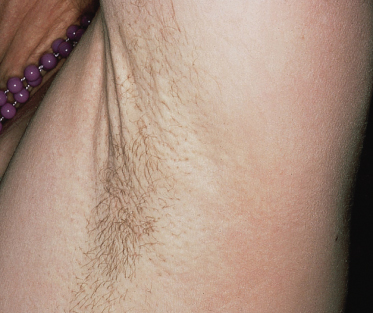
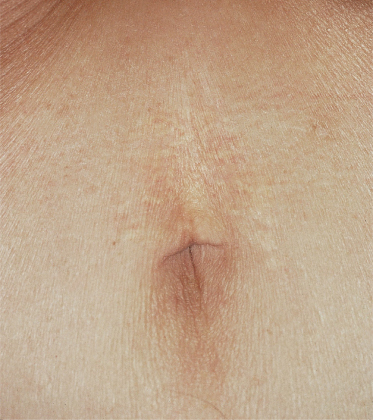
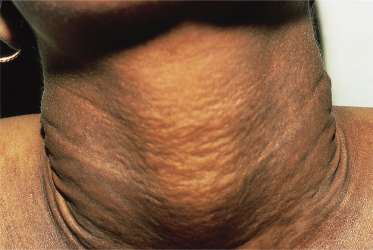
The skin lesions of PXE are said to resemble cobblestones or plucked chicken skin. They have also been called ‘xanthoma-like’ because of their yellowish colour. The skin lesions typically begin on the neck (Fig. 144.3). The next most commonly involved sites are the axillae (Fig. 144.4), but any flexural area can be affected, including the skin superior to the umbilicus (Fig. 144.5). Wrists are less commonly affected. Skin lesions begin as yellow macules that can develop into yellow papules. In more severe cases, papules become confluent to form yellow plaques that simulate plane xanthomas. In very severe cases, patients develop redundant folds of skin in flexural areas (Fig. 144.6) and skin can be strikingly hyperextensible. In patients who are severely affected, the sagging of facial skin can be striking. This results in a ‘hound dog’ appearance that has been seen in patients with cutis laxa.
Characteristic folds in the chin have recently been described in a high proportion of patients with PXE. Although these can occur with normal ageing, they are often seen in PXE patients in their teens and twenties (Fig. 144.7) [54].
The skin lesions of PXE exhibit a Koebner phenomenon, having a tendency to develop within scars. This has been used diagnostically in that biopsy of scars may aid in the diagnosis of PXE in patients without clinically apparent skin lesions [16]. Similarly, in patients with angioid streaks and accelerated cardiovascular disease who do not have skin lesions, biopsy of intertriginous areas such as the neck or axillae can establish a diagnosis of PXE [15].
Skin lesions can occasionally be quite subtle. In one patient examined at the Mount Sinai Medical Center in New York City, PXE was found incidentally in normal-appearing skin around an excised naevus. The patient was subsequently found to have asymptomatic angioid streaks and mild skin lesions on the neck and in the axillae, groin and periumbilical area.
Mucosal lesions develop in many patients. They are easily seen on the mucosal aspect of the lower lip (see Fig. 144.7) and under the tongue. Histological evidence of PXE has been found in rectal mucosa [55], and a patient has been reported with lesions of PXE on the dorsal aspect of the penis [12].
Variants
Perforation of calcified elastic fibres through the epidermis in patients with PXE has been called ‘perforating pseudo-xanthoma elasticum’ [56]. There have been many reports of elastosis perforans serpiginosa occurring in patients with PXE, and it is likely that many of these patients in fact had perforating PXE [57-59]. Although extrusion of dermal material is common to all the perforating disorders, perforating PXE is clearly distinct from the other disorders in that the extruded material consists of calcified elastic fibres.
Another unusual form of PXE has been called ‘localized acquired cutaneous pseudo-xanthoma elasticum’ [60]. This condition appears to be more common in obese, multiparous black females, several of whom have had hypertension. Skin lesions usually occur on the abdomen, particularly in the periumbilical area. In several instances, perforation of the epidermis with dermal extrusion of calcified elastic fibres has been reported [61]. The reported cases have been sporadic without any family history of PXE, and the patients do not generally have other stigmata of PXE, although there has been a solitary report of a black woman with chronic renal failure who developed perforating PXE and was found to have angioid streaks [62]. Neldner & Martinez-Hernandez [60] have also reported a multiparous black woman with cirrhosis who was thought to have localized acquired PXE with skin lesions on the breasts as well as the abdomen.
Ocular Manifestations
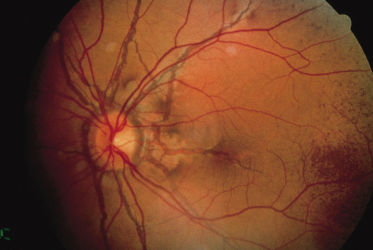
Angioid streaks, the ocular hallmark of PXE, represent breaks in Bruch’s membrane, an elastic tissue-containing membrane of the retina that can become calcified and crack. Angioid streaks appear as blood vessel-like lines that are grey or brown in darkly pigmented individuals (Fig. 144.8) and red in fair-skinned patients. They often encircle the optic disc in a peripapillary distribution or radiate out from the optic disc. Their size is often similar to that of retinal vessels but they can occasionally be much wider than retinal vessels.
Although they have been reported in patients with many different conditions, angioid streaks are most closely associated with PXE. The large majority of adults with PXE have angioid streaks [12]. Because the skin lesions of PXE can be subtle or inapparent [15,16], it is possible that many of the patients reported with angioid streaks may have occult PXE. Angioid streaks have also been reported in several patients with Paget disease and sickle cell anaemia [63,64].
In patients with PXE, angioid streaks often develop in the teenage years. Other ocular features of PXE include mottled ‘peau d’orange’, which consists of mottled pigmentation of the retinal pigment epithelium, and drusen, which appear as small hypopigmented holes in Bruch’s membrane. The development of subretinal neovascularization in these patients can lead to haemorrhage, scarring and loss of vision. If haemorrhage involves the macular area, patients may develop loss of central vision and can become legally blind. Peripheral vision is maintained, however, so that patients do not become totally blind.
Cardiovascular Manifestations
Just like other elastin-containing tissues, the internal elastic lamina of arteries can become calcified. Calcification of arteries can be seen on simple radiographs in up to one-third of patients [12]. Reduced peripheral pulses commonly result from arterial calcification in patients with PXE. Intermittent claudication, presumably due to reduced circulation, has been reported in up to 30% of patients [12]. Claudication has been reported as early as the age of 9 years in a patient with PXE [12]. Intermittent claudication of the arms can also occur and may present as aching, weakness or fatigue on upper extremity exertion. Occlusion of vessels has been demonstrated in arteriograms of the arms [53].
Calcification of the coronary arteries can lead to accelerated cardiovascular disease with symptoms and complications simulating arteriosclerosis. There have been patients with angina or myocardial infarctions in the teenage years. A patient was reported who developed angina pectoris at the age of 11 years and underwent three-vessel coronary artery bypass graft surgery at the age of 18 years [10]. An Australian journal reported three teenage patients with PXE who died of myocardial infarctions, leading the authors to call this disease the ‘sudden death syndrome’ [65].
The severity of skin lesions does not correlate with the development of cardiovascular disease. At the Mount Sinai Medical Center, the authors have seen four patients who developed cardiovascular disease at an early age in the absence of other risk factors for atherosclerosis. All had angioid streaks and histological evidence of PXE but no skin lesions [15]. This has led to the suggestion that any patient with unexplained accelerated heart disease be examined for PXE. Examination for angioid streaks by a retina specialist and skin biopsy of normal-appearing axillary skin or scars may reveal the diagnosis in patients without skin lesions [16].
Other cardiovascular complications of PXE include a restrictive cardiomyopathy reported in one patient [66] and cardiac valvular abnormalities, which are common. Heart valves contain elastic tissue, so it is not surprising that they are affected in patients with this disease. A number of cardiac valvular abnormalities can occur, including fibrous thickening of the atrioventricular valves and mitral valve prolapse. The latter abnormality occurs in approximately two-thirds of patients [67]. Abnormal diastolic dysfunction has been found in less than half of PXE patients without clinical heart disease [68]. These changes may be due to preclinical involvement of coronary artery and endocardial disease or may be the direct consequence of ultrastructural defects of the elastic tissue in the heart. Hypertension has been reported in the region of 9–48% of patients, depending on the series of patients studied [53,69]. It is clear that hypertension increases with age. However, two sisters with PXE have been reported: one died at the age of 10 years of complications of hypertension and the other had elevated blood pressure at the age of 6 years [70]. Several additional reports of hypertension occurring in the teenage years or earlier have been published [71,72]. Elevation of blood pressure has been attributed to narrowing of the renal artery in at least some cases [73,74].
In The Netherlands, many PXE patients were found to have a specific premature truncation variant of ABCC6 known as R1141X. Because of the known association between PXE and accelerated heart disease, a case–control study was performed in which 441 patients with coronary artery disease who were under the age 50 years were compared with 1057 age- and sex-matched control subjects who did not have coronary artery disease. Patients with coronary artery disease were 4.2 times more likely to have the R1141X mutation than the control subjects, lending additional support to a role for this gene in the cardiovascular complications of PXE [75].
Other Systemic Manifestations
In some patients, calcification of the arteries will lead to cracking of the blood vessel and haemorrhage. Bleeding has been reported in many sites, including the uterus, nose, joints, gastrointestinal tract and others. Gastrointestinal bleeding develops in almost 10% of patients [12,69]. In some women, gastrointestinal bleeding occurs during pregnancy [76]. When radiographic and endoscopic examinations are undertaken, the source of bleeding is rarely found [12]. Fortunately, bleeding is often minor and self-limited, but occasionally partial gastrectomy is required.
Other consequences of arterial calcification include central nervous system complications such as stroke and dementia. These are attributed to calcification of cerebral arteries and reduced circulation or intracranial haemorrhage [77,78].Visceral calcifications are part of the phenotype of PXE. Pulmonary calcification has been noted in isolated foci on chest radiographs in a few patients with PXE [79,80]. It is quite interesting that pulmonary calcification is minor and not associated with clinical sequelae in patients with PXE, even though the lungs contain elastic tissue. A report of 17 PXE patients and 17 heterozygous carriers showed calcifications in the liver, kidneys and spleen detected in 59% of the patients and in 23.5% of healthy carriers [81]. Male patients, including young boys, can present with bilateral testicular microlithiasis [82].
Differential Diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree