* A few rare cases with associated features have been reported.
AD, autosomal dominant; AR, autosomal recessive; CHACS, curly hair–acral keratoderma–caries syndrome; CEDNIK, cerebral dysgenesis, neuropathy, ichthyosis and palmoplantar keratoderma syndrome; HOPP, hypotrichosis–osteolysis–periodontitis–palmoplantar keratoderma syndrome; PPK, palmoplantar keratodermas.
Table 120.2 Proposal for a biological classification of PPKs with a known gene defect
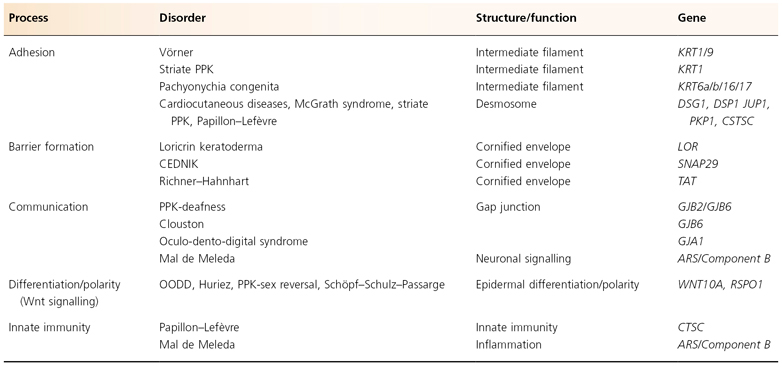
CEDNIK, cerebral dysgenesis, neuropathy, ichthyosis and palmoplantar keratoderma syndrome; OODD, odonto-onycho-dermal dysplasia; PPK, palmoplatar keratoderma.
An integrated approach is necessary for a correct diagnosis. The classification in this chapter is necessarily provisional and must be modified continually as new information becomes available. As a result, several entries have been updated and others removed since the last edition. New syndromes that have been described recently have been added. Note that disorders caused by deficiencies of desmosomal proteins, such as Naxos disease and Carvajal-Huerta syndrome (the cardiocutaneous diseases), can also be grouped with the palmoplantar keratoses. Because they are reviewed in another chapter (Chapter 127), these disorders will not be discussed further here.
The MIM numbers accompanying several of the diseases refer to the online version of McKusick’s Online Mendelian Inheritance in Man (OMIM) [8]. If the number is preceded by a ‘#’, the causative gene defect is known.
References
1 Franceschetti A, Schnyder UW. Versuch einer klinisch-genetischen Klassifikation der hereditären Palmoplantarkeratosen unter Berücksichtigung assozierter Symptome. Dermatologica 1960;120:154–78.
2 Schnyder UW, Klunker W. Erbliche verhornungsstörungen der Haut. In: Gottron HA, Schnyder UW (eds) Handbuch der Haut- und Geschlechtskrankeiten. Berlin: Springer, 1966: 861–961.
3 Greither A. Erbliche Palmoplantarkeratosen. Hautarzt 1977;28:395–403.
4 Voigtländer V, Schnyder UW. Palmoplantarkeratosen. In: Korting GW (ed) Dermatologie in Praxis und Klinik. Stuttgart: Thieme Verlag, 1980: 26–36.
5 Salamon T. An attempt at classification of inherited disorders of keratinization localized mainly, not exclusively on the palms and soles. Dermatol Monatsschr 1986;172:601–5.
6 Lucker GPH, van de Kerkhof PCM, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol 1994;131:1–14.
7 Stevens HP, Kelsell DP, Bryant SP et al. Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmo-plantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Arch Dermatol 1996;132:640–51.
8 OMIM. Online Mendelian Inheritance in Man: available via www.ncbi.nlm.nih.gov/omim/.
Diffuse Hereditary Palmoplantar Keratodermas Without Associated Features
Palmoplantar Keratoderma Cum Degeneratione Granulosa Vörner (MIM #144200, KRT1)
Syn.
- Epidermolytic palmoplantar keratoderma
- Palmoplantar keratoderma type Thost
- Palmoplantar keratoderma type Unna
- Norbotten-type palmoplantar keratoderma
History.
In 1909, Vörner [1] described a diffuse PPK that was histologically characterized by epidermolytic hyperkeratosis (granular degeneration or acanthokeratolysis). Küster & Becker [2] re-examined the family described by Thost clinically and histologically and demonstrated that the PPK in this family was identical to that described by Vörner. In fact, Vörner described the PPK of Unna and Thost. In 1994, Reis et al. [3] elucidated the cause of this disease by identifying mutations in the gene coding for keratin 9. PPK type Vörner is probably the most frequent form of PPK.
Molecular Pathology.
This PPK is an autosomal dominant disease and is caused by point mutations in the gene coding for keratin 9, which lies on chromosome 17q12–21 [3]. Keratin 9 is expressed in the suprabasal compartment of the epidermis and is specific for the skin on the palms and soles [4].
The pertinent molecular pathology of keratin disorders is reviewed in Chapter 117; here it suffices to say that the vast majority of pathogenic mutations in keratins are dominant and probably disrupt intermediate filament assembly, leading to cytoskeletal dysfunction [5]. In contrast to what was previously thought, the mechanical functions of the intermediate filament cytoskeleton in keratinoctyes do not suffice to explain the epithelial phenotype [6]. Disruption of various signalling pathways and endoplasmic reticulum stress are likely to also contribute (see Chapter 117). The hyperkeratosis is probably best understood as a response to cellular stress that is not purely mechanical.
Pathology.
We do not recommend biopsy for a clinically evident Vörner type PPK. When in doubt as to the diagnosis, we prefer mutation analysis because the microscopic findings in Vörner are not specific. Epidermolytic hyperkeratosis is also found, for example, in bullous congenital ichthyosiform erythroderma of Brocq, desmosomal disorders such as McGrath syndrome and in linear epidermolytic epidermal naevi. PPK nummularis/hereditary painful callosities is another PPK characterized by epidermolytic hyperkeratosis [7]. Occasionally a biopsy may be required and then epidermolytic hyperkeratosis should be specifically sought. It is characterized by perinuclear vacuolization, large keratohyaline granules, clumping of tonofilaments, cellular degeneration in spinous and granular cells and sometimes blister formation [8]. The abnormalities are sometimes subtle and careful light or even electron microscopical examination of several biopsies can be necessary [9].
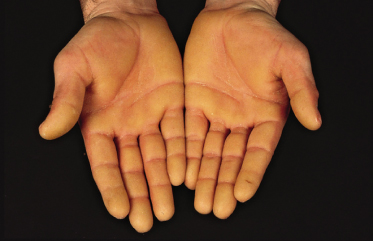
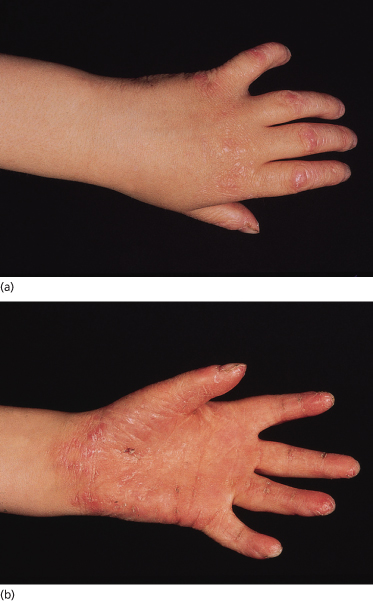
Clinical Features (Fig. 120.1).
The typical Vörner hyperkeratosis is diffuse, non-transgredient and has a brown-yellow coloir with sometimes a greyish hue. There can be accentuation on the pressure points. The hyperkeratosis is smooth and this should be specifically looked for. Sometimes there are pits in it, particularly on the feet. This may be indicative of a Corynebacterium minutissimum infection causing keratoma sulcatum. A Wood’s lamp therefore is helpful when examining patients with keratoderma. Knuckle pads have been reported [10]. Onset is in early childhood, first on the palms when the child starts to crawl. Rarely, symptoms may appear in adulthood.
Differential Diagnosis.
Palmoplantar keratoderma type Vörner can sometimes be differentiated from the other diffuse types of PPK by the histopathological characteristics of epidermolytic hyperkeratosis. Usually, associated features and the phenotype will suffice to establish the diagnosis. This can be difficult in the case of epidermolytic ichthyosis where some KRT1 mutations can result in very mild or even absent blistering in friction areas, associated with a palmoplantar keratoderma that is indistinguishable from Vörner type. Considering that keratin 1 pairs with keratin 9 in palmoplantar skin, this is not suprising. Mutation analysis is required to establish the diagnosis in such cases.
Treatment.
Patients can be helped by the application of keratolytics such as salicylic acid (contraindicated in very young children), urea, lactic acid and propylene glycol in white soft paraffin, as a gel or an ointment. Occlusion under polyethylene is often more beneficial. Topical retinoids have some effect but systemic retinoids such as acitretin are more effective [11]. Because of possible side-effects and teratogenicity, systemic retinoids should be prescribed only after serious consideration. Fungal and bacterial superinfections should be treated adequately, if necessary using systemic therapy. Systemic retinoids may cause excessive peeling and even blistering. Many patients eventually discontinue their treatment because they have come to depend on the mechanical or thermal barrier that their hyperkeratosis provides. For example, one of our patients is a homemaker whose PPK allows her to take dishes out of a hot oven without wearing mitts.
References
1 Vörner H. Zur Kenntnis des Keratoma hereditarium palmare et plantare. Arch Derm Syph (Berlin) 1901;56:3–31.
2 Kuster W, Becker A. Indication for the identity of palmoplantar keratoderma type Unna–Thost with type Vorner. Thost’s family revisited 110 years later. Acta Derm Venereol 1992;72(2):120–2.
3 Reis A, Hennies HC, Langbein L et al. Keratin 9 gene mutations in epidermolytic palmoplantar keratoderma (EPPK). Nat Genet 1994;6(2):174–9.
4 Korge BP, Krieg T. The molecular basis for inherited bullous diseases. J Mol Med 1996;74(2):59–70.
5 Smith F. The molecular genetics of keratin disorders. Am J Clin Dermatol 2003;4(5):347–64.
6 Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res 2007;313(10):2021–32.
7 Wachters DH, Frensdorf EL, Hausman R et al. Keratosis palmoplantaris nummularis (‘hereditary painful callosities’). Clinical and histopathologic aspects. J Am Acad Dermatol 1983;9(2):204–9.
8 Moriwaki S, Tanaka T, Horiguchi Y et al. Epidermolytic hereditary palmoplantar keratoderma. Histologic, ultrastructural, protein-chemical and DNA analyses in two patients. Arch Dermatol 1988;124(4):555–9.
9 Kuster W, Zehender D, Mensing H et al. [Vorner keratosis palmoplantaris diffusa. Clinical, formal genetic and molecular biology studies of 22 families.] Hautarzt 1995;46(10):705–10.
10 Nogita T, Nakagawa H, Ishibashi Y. Hereditary epidermolytic palmoplantar keratoderma with knuckle pad-like lesions over the finger joints. Br J Dermatol 1991;125(5):496.
11 Happle R, van de Kerkhof PC, Traupe H. Retinoids in disorders of keratinization: their use in adults. Dermatologica 1987;175(suppl 1):107–24.
Mal de Meleda (MIM #248300, ARS Component B)
Syn.
- Meleda disease
- Mal de Mljet
- Keratosis extremitatum hereditaria transgrediens et progrediens
- Keratoderma palmoplantaris transgrediens
History.
This rare PPK was first described by Hovarka & Ehlers in 1897 and further delineated by, among others, Brunner & Fuhrman in 1950 and Franceschetti et al. in 1972 [1–3]. The disorder takes its name from the Croatian island of Mljet (Meleda), where it is unusually frequent as a result of inbreeding.
Molecular Pathology.
The inheritance is autosomal recessive. Fischer et al. [4] demonstrated that mal de Meleda is associated with mutations in the ARS gene encoding the SLURP-1 (for secreted Ly6/uPAR-related protein-1) protein, a member of the Ly-6/uPAR protein family and homologous to snake venom and frog neurotoxins. The gene has been mapped to chromosome 8q24.3 [5]. The structure of the protein suggests that it may act as a neuromodulator in skin. Recent data support this notion, showing that SLURP-1 modulates the action of acetylcholine receptors present in keratinocytes [6,7]. It also has a pro-apoptotic activity [7] and there are indications that it is involved in regulating tumour necrosis factor (TNF)-α release by skin macrophages [6]. Involvement of acetylcholine signalling in the disorder is conceptually consistent with the hyperhidrosis, although the relation is not yet understood. Relatively few different mutations have been found in patients of Moroccan and Croatian descent, suggesting a founder effect in the Mediterranean basin, i.e. the mutation arose in a common ancestor of populations that later migrated and was maintained by inbreeding [4]. Our own recent findings suggest that the same applies to mainland Europe, as we find one mutation in Holland that has also been reported in Germany [8,9].
Pathology.
The histological findings are not very informative and consist of hyperkeratosis with some parakeratosis and a marked acanthosis. A prominent perivascular mononuclear infiltrate is often present.
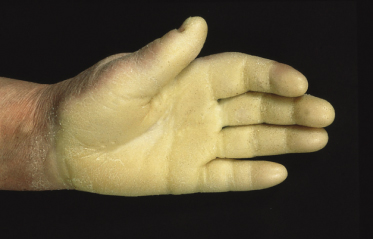
Clinical Features (Fig. 120.2).
Mal de Meleda is characterized by a diffuse, thick, white to yellow, macerated-looking hyperkeratosis with a prominent transgredient erythematous border. The thick hyperkeratosis may lead to flexion contractures. The disease has its onset in early infancy and follows a progressive course with extension onto the dorsal surfaces. Constricting bands surrounding the digits are typical [10], resulting rarely in spontaneous amputation of the digits [11]. Concomitant lesions can be found at other sites, especially the elbows and knees [4]. Perioral erythema and hyperkeratosis may be present [12], resembling the clinical features of Olmsted’s syndrome. Hyperhidrosis of the affected parts, with maceration of the hyperkeratotic masses, bacterial or fungal superinfection and the consequent production of a rancid odour, is pronounced. Examination with a Wood’s lamp is recommended. Nail changes (koilonychia, nail thickening, subungual hyperkeratosis) can be present. Onset is in infancy or early childhood.
Differential Diagnosis.
There exists a somewhat similar but milder disorder without the thick yellow-white hyperkeratosis, called Nagashima type PPK [13]. It seems not to be associated with ARS component B mutations [14]. In young children the hyperhidrosis is less prominent. It may be difficult to distinguish Meleda from either Vörner or Olmsted PPK, in particular in very young children. Mutation analysis or long-term follow-up may be needed.
Treatment.
A good response to retinoids has been reported [15], especially a reduction in the hyperkeratosis, but the erythema may become more prominent. Any bacterial or fungal superinfection should be treated, if possible with topical agents. The distinctive odour often improves after such interventions. The hyperhidrosis might result from alterations in acetylcholine signalling. Because SLURP-1 potentiates the acetylcholine receptor, botulism toxin may not be helpful. Topical agents such as aluminium salts are probably more useful.
References
1 Hovorka O, Ehlers E. Meledakrankheit. Arch Derm Syph 1896;34:51.
2 Brunner MJ, Fuhrman DL. Mal de Meleda. Report of a case and results of treatment with vitamin A. Arch Derm Syph 1950;61:820–3.
3 Franceschetti AT, Reinhart V, Schnyder UW. La maladie de Meleda. J Genet Hum 1972;20:267–96.
4 Fischer J, Bouadjar B, Heilig R et al. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum Mol Genet 2001;10(8):875–80.
5 Fischer J, Bouadjar B, Heilig R et al. Genetic linkage of Meleda disease to chromosome 8qter. Eur J Hum Genet 1998;6(6):542–7.
6 Chimienti F, Hogg RC, Plantard L et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet 2003;12(22):3017–24.
7 Arredondo J, Chernyavsky A, Webber R et al. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol 2005;125(6):1236–41.
8 Eckl KM, Stevens HP, Lestringant GG et al. Mal de Meleda (MDM) caused by mutations in the gene for SLURP-1 in patients from Germany, Turkey, Palestine and the United Arab Emirates. Hum Genet 2003;112(1):50–6.
9 Nellen RG, van Geel M, Steijlen PM et al. Compound heterozygosity for ARS component B mutations in a Dutch patient with mal de Meleda. Br J Dermatol 2009;160(4):878–80.
10 Degos MMR, Delort J, Charlas J. Ainhum avec kératodermie palmoplantaire. Bull Soc Fr Dermatol Syphiligr 1963;70:136–8.
11 Lestringant GG, Hadi SM, Qayed KI et al. Mal de Meleda: recessive transgressive palmoplantar keratoderma with three unusual facultative features. Dermatology 1992;184(1):78–82.
12 Bouadjar B, Benmazouzia S, Prud’homme JF et al. Clinical and genetic studies of 3 large, consanguineous, Algerian families with Mal de Meleda. Arch Dermatol 2000;136(10):1247–52.
13 Kabashima K, Sakabe J, Yamada Y et al. ‘Nagashima-type’ keratosis as a novel entity in the palmoplantar keratoderma category. Arch Dermatol 2008;144(3):375–9.
14 Van Steensel M, van Geel M, Steijlen P. Mal de Meleda without mutations in the ARS coding sequence. Eur J Dermatol 2002;12(2):129–32.
15 Van de Kerkhof PC, van Dooren-Greebe RJ, Steijlen PM. Acitretin in the treatment of mal de Meleda. Br J Dermatol 1992;127(2):191–2.
Diffuse Hereditary Palmoplantar Keratodermas with Associated Features
Loricrin Keratoderma (MIM #604117, LOR)
Syn.
- Progressive symmetrical erythrokeratoderma
- Camisa variant of Vohwinkel syndrome
History.
This rare disorder was originally (and erroneously) reported by Camisa et al. as a variant of Vohwinkel syndrome [1]. The first LOR mutation defining this disorder as a separate entity was reported in 1996 by Maestrini et al. who likewise wrongly diagnosed their patient [2]. Further confusion resulted from a subsequent report describing the finding of loricrin mutations in progressive symmetrical erythrokeratoderma – PSEK [3]. However, PSEK is a manifestation of erythrokeratoderma variabilis (EKV), a gap junction disorder caused by mutations in the GJB3 or GJB4 genes, and is quite different in appearance [4]. Loricrin mutations are therefore associated with a recognizable entity that we will refer to as ‘loricrin keratoderma’. Note that it is currently also classified as an ichthyosis.
Molecular Pathology.
Loricrin keratoderma is an autosomal dominant trait caused by insertion mutations in the LOR gene, leading to frameshifts resulting in extension of the loricrin peptide by 22 amino acids. The gene is in the epidermal differentiation complex on chromosome 1q21. Three different insertions have been reported to date, all resulting in the same extension. Loricrin is a major component of the cornified envelope and contains three glycine-rich domains interspersed with glutamin-rich motifs. Thus, loricrin is thought to function as a major component of transglutaminase-formed cross-links in the cornified envelope [5]. The mutations disrupt normal loricrin–loricrin interactions and abolish several transglutaminase targets [6], putatively resulting in abnormal cornified envelope assembly. Furthermore, the mutations cause arginine-containing nuclear localization signals to appear in the glycine-rich domain of the mutant loricrin, which translocates to the nucleus [6]. Mouse studies have shown that this interferes with late stages of epidermal differentiation [7]. How this results in corneal envelope dysfunction is not clear. Several other proteins are more extensively cross-linked in loricrin keratoderma, potentially mitigating the barrier dysfunction (Peter Elias, personal communication) [7,8].
Pathology.
This is wholly unspecific, showing hyperparakeratosis, psoriasiform epidermal hyperplasia and a perivascular lymphocytic infitrate. Electron microscopy is more informative, showing intranuclear granules in upper granular layer cells and a conspicuous transitional cell layer. The cornified envelope is abnormal, containing lipid droplets and a lack of increase in thickness of cell envelopes [9].
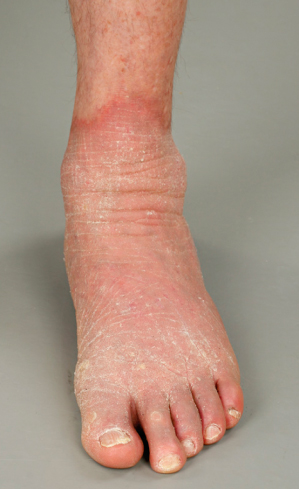
Clinical Features (Fig. 120.3).
Loricrin keratoderma typically manifests in early childhood with non-migratory erythematous sharply demarcated plaques of thickened skin over the extensor surfaces of large joints. The associated palmoplantar keratoderma is usually diffuse, transgredient and can be quite erythematous. Pseudo-ainhum and starfish-like extension onto the wrist and ankles can occur; starfish-like keratoses can affect the trunk. Flaky hyperkeratosis can be present. Sometimes a slight truncal ichthyosis is seen. Importantly, hearing loss is absent.
Differential Diagnosis.
The disorder must be distinguished from Vohwinkel syndrome. The keratoderma in the latter is limited to the hands and feet and can show smooth palmoplantar hyperkeratosis. Knuckle pads are more often present in Vohwinkel syndrome. The clearest distinction obviously is the hearing loss. EKV can be a consideration but the migratory nature of hyperkeratotic (not keratodermic) erythematous rather flat plaques usually distinguishes this particular disorder. A rare disorder called KLICK syndrome (for keratosis linearis with ichthyosis congenita and sclerosing keratoderma) can be potentially confused with loricrin keratoderma. KLICK is genetically distinct, so when in doubt, mutation analysis can be helpful [10]. In general, KLICK is characterized by the presence of linear keratoderma, sometimes with a starfish configuration, in the flexures. These are usually spared in loricrin keratoderma. The extensor surfaces show keratodermic erythematous plaques. There is a generalized mild ichthyosis and a diffuse PPK without pseudo-ainhum.
Treatment.
Topical keratinolytic agents are of limited use. Emollients can be helpful. It is worth trying topical vitamin D3 or retinoids but their effect in our experience is limited. Systemic retinoids can be tried but likewise are not very effective in reducing the keratoderma.
References
1 Camisa C, Rossana C. Variant of keratoderma hereditaria mutilans (Vohwinkel’s syndrome). Treatment with orally administered isotretinoin. Arch Dermatol 1984;120(10):1323–8.
2 Maestrini E, Monaco AP, McGrath JA et al. A molecular defect in loricrin, the major component of the cornified cell envelope, underlies Vohwinkel’s syndrome. Nat Genet 1996;13(1):70–7.
3 Ishida-Yamamoto A, McGrath JA, Lam H et al. The molecular pathology of progressive symmetric erythrokeratoderma: a frameshift mutation in the loricrin gene and perturbations in the cornified cell envelope. Am J Hum Genet 1997;61(3):581–9.
4 Van Steensel MA, Oranje AP, van der Schroeff JG et al. The missense mutation G12D in connexin30.3 can cause both erythrokeratodermia variabilis of Mendes da Costa and progressive symmetric erythrokeratodermia of Gottron. Am J Med Genet A 2009;149A(4):657–61.
5 Hitomi K. Transglutaminases in skin epidermis. Eur J Dermatol 2005;15(5):313–19.
6 Ishida-Yamamoto A. Loricrin keratoderma: a novel disease entity characterized by nuclear accumulation of mutant loricrin. J Dermatol Sci 2003;31(1):3–8.
7 Koch PJ, de Viragh PA, Scharer E et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol 2000;151(2):389–400.
8 Schmuth M, Fluhr JW, Crumrine DC et al. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis). J Invest Dermatol 2004;122(4):909–22.
9 Ishida-Yamamoto A, Takahashi H, Iizuka H. Loricrin and human skin diseases: molecular basis of loricrin keratodermas. Histol Histopathol 1998;13(3):819–26.
10 Van Steensel MA, van Geel M, Steijlen PM. A new type of erythrokeratoderma. Br J Dermatol 2005;152(1):155–8.
Keratosis Linearis with Ichthyosis Congenita and Sclerosing Keratoderma (KLICK) Syndrome (MIM #601952)
History.
In 1989, Pujol et al. described this rare entity in four Spanish siblings [1]. It was later further defined by Vahlquist et al. in an isolated case [2]. They coined the ‘KLICK’ acronym. A new case from Holland has been reported recently [3].
Molecular Pathology.
Inheritance is autosomal recessive. All patients reported to date have the same causative mutation affecting the 5’ UTR of the POMP gene on chromosome 13q. POMP codes for a component of the proteasome which is the cellular machine for breaking down degraded, misfolded or otherwise non-functional proteins. In KLICK, proteasomal distribution in the upper epidermis is altered. As a result, protein breakdown is disturbed and endoplasmic reticulum (ER) stress ensues which then causes hyperkeratosis. Thus, while genetically distinct, the pathogenesis of KLICK is comparable to that of PPK Vörner. Conceivably therefore, ER stress might represent a novel therapeutic target for (selected) PPKs [4].
Pathology.
Unspecific, showing orthohyperkeratosis and epithelial hyperplasia with hypergranulosis. Electron microscopy shows numerous large keratohyaline granules in superficial keratinocytes.
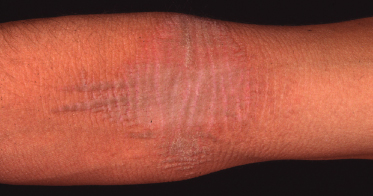
Clinical Features (Fig. 120.4).
The disorder typically presents with generalized erythroderma and fine scaling in early childhood. Erosions can also occur. Later, a diffuse palmoplantar keratoderma with constricting bands develops in addition to linear or starfish-shaped keratoderma affecting both flexor and extensor sides of large joints. There are no other anomalies.
Differential Diagnosis.
The KLICK syndrome must be primarily distinguished from loricrin keratoderma. A good clue to the diagnosis is the palmoplantar keratoderma, which in KLICK is smooth and shows no starfish-like extensions. Likewise, loricrin keratoderma can give starfish-like keratosis on truncal locations, which KLICK will never do. In early childood KLICK may be mistakenly diagnosed as a form of ichthyosis or EKV.
Treatment.
Topical emollients and keratolytics are not very effective. Acitretin can give satisfactory improvement of both the ichthyosis and the keratoderma [3].
References
1 Pujol RM, Moreno A, Alomar A et al. Congenital ichthyosiform dermatosis with linear keratotic flexural papules and sclerosing palmoplantar keratoderma. Arch Dermatol 1989;125(1):103–6.
2 Vahlquist A, Ponten F, Pettersson A. Keratosis linearis with ichthyosis congenita and sclerosing keratoderma (KLICK syndrome): a rare, autosomal recessive disorder of keratohyaline formation? Acta Derm Venereol 1997;77(3):225–7.
3 Van Steensel MA, van Geel M, Steijlen PM. A new type of erythrokeratoderma. Br J Dermatol 2005;152(1):155–8.
4 Dahlqvist J, Klar J, Tiwari N et al. A single-nucleotide deletion in the POMP 5’ UTR causes a transcriptional switch and altered epidermal proteasome distribution in KLICK genodermatosis. Am J Hum Genet 2010 Apr 9;86(4):596–603.
Palmoplantar Keratoderma with Scleroatrophy (Huriez Syndrome) (MIM 181600)
History.
The syndrome was first described by Huriez et al. [1–3] in two families resident in the north of France. So far, eight families [1–9] and one case report of an affected individual [10] have been published.
Molecular Pathology.
The condition is inherited in an autosomal dominant manner. There is a report of linkage to chromosome 4q23 [11]. This region contains several candidate genes. However, pathogenic mutations have so far not been detected [unpublished data]. It is notable that a case of ‘Huriez’ with 46,XX sex reversal has been published [12]. It was recently found that this constellation is caused by mutations in the RSPO1 gene, coding for R-Spondin1 [13]. R-Spondins are a family of proteins that facilitate Wingless signalling and, as will be further discussed below, dysfunction of Wingless signalling in the skin is associated with abnormalities of epidermal differentiation, including malignancy [14] and disturbances of nail development [15]. It is therefore tempting to speculate that Huriez syndrome is caused by abnormal, possible overactive, Wingless signalling in palmoplantar skin.
Pathology.
The epidermal Langerhans cells are virtually absent from the involved skin only [8]. Histopathological changes are aspecific, resembling those found in odonto-onychodermal dysplasia and 46,XX sex reversal/PPK syndrome.
Clinical Features (Fig. 120.5).
The disease usually starts at birth or during early childhood. The PPK consists of a discrete, sometimes lamellated hyperkeratosis with atrophy, diffusely covering especially the palmar skin. The plantar skin usually displays less severe involvement. Atrophic plaques may be found on the dorsa of the hands and fingers. The affected skin usually is erythematous. Obligatorily associated features are sclerodactyly, brachydactyly and nail changes. The sclerodactyly strongly resembles scleroderma, although the genetic inheritance, absence of systemic symptoms, lack of vasomotor phenomena, appearance of the initial symptoms at birth or in early childhood and absence of progression during adulthood enable a differentiation. Nail changes consist of aplasia, ridging and clubbing. Hypohidrosis is associated in half of the cases. The distinctive feature of this syndrome is the risk of development of squamous cell carcinoma on the affected skin, which may occur as early as the third or fourth decade. These have been observed in an average of 13% of patients. Four patients have died from metastasis of the cutaneous malignancy. Other abnormalities have not been described, although the authors are aware of delayed puberty in a male (46,XY) patient [unpublished].
Treatment.
Until now, only one patient with Huriez syndrome has been treated with retinoids, for 5 years. This patient did not develop any further squamous cell carcinomas during this period [16]. Theoretically, topical vitamin D3 might be of benefit.
References
1 Huriez CL, Agache P, Bombart M et al. Epithéliomas spinocellulaires sur atrophie cutanée congénitale dans deux familles à morbidité cancéreuse élevée. Bull Soc Fr Dermatol Syphiligr 1963;70:24–8.
2 Huriez CL, Agache P, Souillart F et al. Scléroatrophie familiale des extrémités avec dégénérescence cellulaires multiples. Bull Soc Fr Dermatol Syphiligr 1963;70:743–4.
3 Huriez CL, Deminati M, Agache P et al. Génodermatose scléro-atrophiante et kératodermique des extrémités. Ann Dermatol Syphiligr 1969;96:135–46.
4 Lambert D, Planche H, Chapuis JL. La génodermatose scléro-atrophiante et kératodermique des extrémités. Ann Dermatol Venereol 1977;104:654–7.
5 Fischer S. La génodermatose scléro-atrophiante et kératodermique des extrémités. Ann Dermatol Venereol 1978;105:1079–82.
6 Shaw M, Formentini E, de Kaminsky AR et al. [Scleroatrophying and degenerative keratodermic genodermatosis of the extremities.] Med Cutan Ibero Lat Am 1978;6(5–6):291–5.
7 Kavanagh GM, Jardine PE, Peachey RD et al. The scleroatrophic syndrome of Huriez. Br J Dermatol 1997;137(1):114–18.
8 Hamm H, Traupe H, Brocker EB et al. The scleroatrophic syndrome of Huriez: a cancer-prone genodermatosis. Br J Dermatol 1996;134(3):512–18.
9 Lucker GP, Zeedijk N, Steijlen PM. The Huriez syndrome. Scleroatrophic palmoplantar keratoderma. Eur J Dermatol 1997;7:155–7.
10 Patrizi A, di Lernia V, Patrone P. Palmoplantar keratoderma with sclerodactyly (Huriez syndrome). J Am Acad Dermatol 1992;26(5 Pt 2):855–7.
11 Lee YA, Stevens HP, Delaporte E et al. A gene for an autosomal dominant scleroatrophic syndrome predisposing to skin cancer (Huriez syndrome) maps to chromosome 4q23. Am J Hum Genet 2000;66(1):326–30.
12 Vernole P, Terrinoni A, Didona B et al. An SRY-negative XX male with Huriez syndrome. Clin Genet 2000;57(1):61–6.
13 Parma P, Radi O, Vidal V et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 2006;38(11):1304–9.
14 Yang SH, Andl T, Grachtchouk V et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat Genet 2008;40(9):1130–5.
15 Bruchle NO, Frank J, Frank V et al. RSPO4 is the major gene in autosomal-recessive anonychia and mutations cluster in the furin-like cysteine-rich domains of the Wnt signaling ligand R-spondin 4. J Invest Dermatol 2008;128(4):791–6.
16 Delaporte E, N’Guyen-Mailfer C, Janin A et al. Keratoderma with scleroatrophy of the extremities or sclerotylosis (Huriez syndrome): a reappraisal. Br J Dermatol 1995;133(3):409–16.
Palmoplantar Hyperkeratosis with Squamous Cell Carcinoma of Skin and Sex Reversal (MIM #610644, RSPO1)
Syn.
- Palmoplantar hyperkeratosis and true hermaphroditism
History.
This possibly unique entity was first reported as Huriez syndrome with 46,XX sex reversal [1,2]. In 2005, four brothers from a consanguineous family, including the patient described by Guerriero et al. [2], were reported as having a 46,XX karyotype, with palmoplantar keratosis and predisposition to squamous cell carcinoma of the skin [3]. Another individual with a similar disorder was subsequently described; both this patient and the previously reported family were from southern Italy [4]. Most recently, a 46,XX woman with true hermaphroditism, dystrophic nails and palmoplantar keratoderma was reported, again in southern Italy [5].
Molecular Pathology.
Homozygosity mapping in the individuals reported in 2005 led to the finding of a homozgous single-nucleotide insertion in the RSPO1 gene coding for the protein R-Spondin1 [6]. In mammals there are at least four R-Spondins. Recent research suggests that these proteins potentiate Wingless (WNT) signalling by binding to the Frizzled/LRP receptor complex that transmits the Wingless signal [7]. 46,XX sex reversal is unusual and poorly understood. However, loss-of-function WNT4 mutations cause the SERKAL syndrome, a rare disorder of, among other symptoms, female sex reversal [8]. Also, it has been known since 1999 that WNT4 is required for female development in mammals [9]. This explains how loss of RSPO1 can lead to female sex reversal. WNT signalling is further involved in dorsal-ventral polarization and determination of nail development (autosomal recessive anonychia, for example, is caused by absence of RSPO4 [10]). From odonto-onychodermal dysplasia (see below) we also know that WNT signalling is required for normal epidermal differentiation and nail development. Furthermore, recent findings firmly imply WNT signalling in skin carcinogenesis [11]. Organotypic culture using RSPO1 -/- keratinocytes showed a profound defect in differentiation, the cells forming a basal cell layer only [6].
Pathology.
The only available description lists hyperkeratosis, vacuolar degeneration of keratinocytes in the upper stratum spinosum and a thickened granular layer with hypergranulosis. There is a photograph illustrating these abnormalities; the overall impression is of a somewhat disorderly differentiation with a smooth orthohyperkeratosis [1].
Clinical Features.
The cardinal feature is that of more or less complete sex reversal in a 46,XX individual with a Huriez-like palmoplantar keratoderma and abnormal nails. The authors would go so far as to suggest that every patient presenting with Huriez-like keratoderma should be examined for signs of sex reversal and be offered karyotyping. The squamous cell carcinomas reported were moderately differentiated. Further abnormalities that have been reported include hypertriglyceridaemia, Leydig cell hyperplasia and loss of teeth in early adulthood due to periodontitis.
Differential Diagnosis.
Huriez syndrome can be distinguished in males by a 46,XY karyotype. The PPK is quite similar but the sclerobrachydactyly that is so striking in Huriez is lacking. Odonto-onycho-dermal dysplasia is distinguished by abnormal tooth development and a much milder PPK.
Treatment.
No causative treatment is available. The sequelae of sex reversal and the squamous cell carcinomas will require a surgical approach. Topical emollients may be of benefit.
References
1 Vernole P, Terrinoni A, Didona B et al. An SRY-negative XX male with Huriez syndrome. Clin Genet 2000;57(1):61–6.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree