12 Evidencebased Medicine for Facial Plastic Surgeons
Introduction
The current century has been described as “the century of the patient.” Dr. David Sackett, considered the father of evidencebased medicine (EBM), described evidencebased medicine as “the explicit and judicious use of current best evidence combined with individual clinical expertise and patient preferences and values in making decisions about the care of individual patients.” 1 Most medical and surgical treatments have benefits and harms. EBM bridges the gap between the evidence and patient values by considering the evidence in the context of patient values and preferences. Concerns about persistent unintentional variations in practice, 2 questions about the trustworthiness of acclaimed research in medicine, 3 and a perceived growth in unnecessary medical services 4 have further galvanized an enthusiasm for ensuring the provision of high value care. The facial plastic surgery community has embraced the implementation of EBM as well, as demonstrated by a trend of increasing levels of evidence of manuscripts in our journal.
But on a practical level, the individual provider may wonder why it is truly important to the facial plastic and reconstructive surgeon to have an appreciation for, and a commitment to, practicing EBM. Within the specialty of facial plastic and reconstructive surgery there are a number of areas considered soft science, where subjective aesthetic results are the primary outcome of interest. However, even within our specialty we have opportunity to provide the highest quality care for our patients by considering the evidence. A first step to practicing in an evidencebased manner is becoming familiar with the language of EBM, and understanding its biases and limitations. 5 This chapter illustrates EBM using a practical, case scenario approach common to the facial plastic and reconstructive surgeon.
The Case
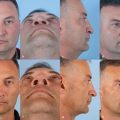
A 45-year-old woman presents with complaints of nasal airway obstruction. She describes it as being bilateral, worse when she is lying flat, and better when she pulls on her cheeks to open her nasal passages. She tells her physician she needs a computed tomography (CT) scan to evaluate for sinusitis, because her primary care physician always checks a CT scan when she has episodes of nasal obstruction. She also states she needs surgery to open her nasal passages. Her colleague with similar problems is doing great after having a septoplasty and nasal valve reconstruction, so the patient would like these surgical procedures, also.
Elements of EBM
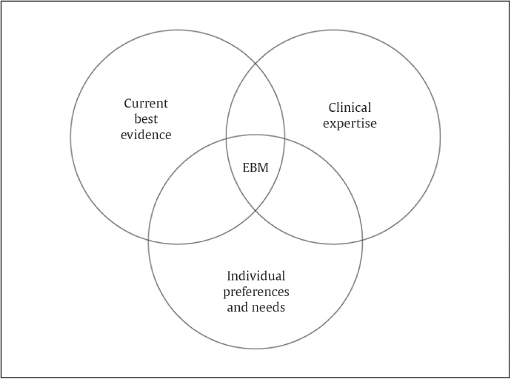
EBM should not be seen as a replacement for the classical teachings of medicine. At its core, the translation of EBM actually requires the consideration of three important concepts: best research evidence available, clinical expertise of the provider, and patient values ( Fig. 12.1 ). 1 , 6 , 7 Imbedded in these concepts is the assumption that a physician adhering to recommendations from a study designed under the postulates of EBM has an adequate comprehension of the level of the evidence in question, possesses the clinical experience to apply the recommendation, and is sensitive to the patient’s self-determination, autonomy, and cultural/spiritual beliefs. 8 These EBM philosophical tenets allow for variations in patient care based on the individual physician–patient encounter and relationship. There is little doubt that scientific recommendations from well-designed studies improve our standard of living and safety of health care. 9 However, the act of applying the science itself is an art that is shaped by compassion, creativity, experience, finely tuned skills, and dedication to one’s craft. EBM provides external information but it does not replace the wisdom and expertise used in applying such information: one hat does not fit all. EBM should not be regarded as cookbook medicine that will replace traditional medicine; translating EBM itself fosters the provision of safe therapies in a unique, patient-tailored manner that fully appreciates the art of medicine. 10 , 11
Current Best Evidence
“Current best evidence” is often mistakenly held synonymous with randomized controlled trials (RCTs) or other studies with high levels of evidence. A detailed description of a commonly used rating system of evidence is explained later in the chapter. A higher level of evidence is a reflection of the rigor involved in the research design to limit bias and scientifically answer questions in a clear fashion. However, one has to be careful to assume that a higher level of evidence for a given clinical question is always better evidence.
Furthermore, there can be an intrinsic bias to the type of intervention that is chosen to be studied; a certain intervention may lend itself well to blinding (patient and/or observer), thereby achieving the goal of attaining higher level of evidence, but the intervention itself may not necessarily be the best intervention for that condition. Third party payers or other stakeholders may then erroneously conclude that this chosen intervention is the “best” intervention when in fact there may be a “better” intervention that can never lend itself to be studied at a level 1 study design.
Physicians who truly strive to understand the utility of EBM will appreciate other levels of evidence as good sources of information for patient care. In fact, well-designed cohort studies may report similar outcomes provided by RCTs. Advancement of knowledge may occur via case reports, case series, cohort studies, and experiments without controls, uncontested expert opinion, or basic science research
Levels of Evidence
Level of evidence (LOE) designations stratify evidence from strongest to weakest based on the basis of susceptibility of the study to bias and the rigor of the study design. A common evidence system is the LOE system from the Oxford Center for Evidence Based Medicine (OCEBM). In this system, the highest evidence is designated by level 1, with the lowest evidence considered level 5 ( Table 12.1 ). 12 The LOE designation is applicable only to clinical studies.
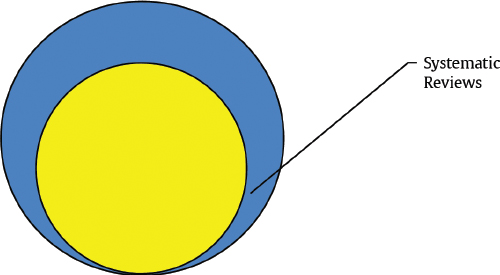
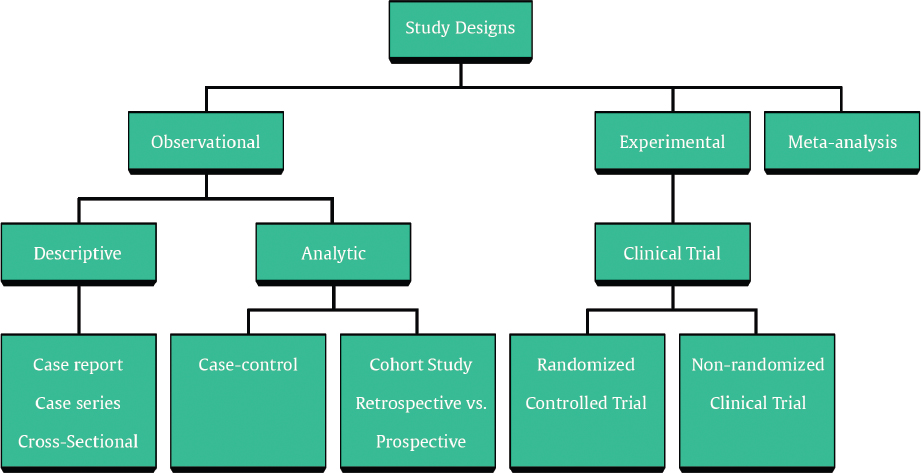
A systematic review is a review conducted according to rigorous scientific research methods designed to minimize bias. Explicit search criteria and data extraction guidelines should be provided for the review to allow for independent verification of the review. With transparent methods designed a priori, systematic review investigations are meant to be reproducible with limited risk of bias and random error. 13 A metaanalysis is a statistical method of combining results from different studies. A metaanalysis can be performed on combined data from a systematic review if the data are deemed similar enough for comparison ( Fig. 12.2 ).
RCTs are considered the best research type because they provide the most rigorous way of determining if a cause and effect relationship exists between treatment and outcome ( Fig. 12.3 ). 14 Where other study designs can show associations between interventions and outcomes, they cannot rule out the possibility that there is an undetected confounding variable that accounts for the relationship. Randomization provides a level of assurance that two groups are comparable in every way except for receipt of the intervention. The intervention and control groups are equivalent in the things we know, and the things we do not know matter.
RCTs are difficult to perform in surgery for a number of reasons, including ethical and patient recruitment concerns, concealment limitations, and the blinding itself. However, methods for minimizing these limitations exist, so surgical RCTs are certainly feasible. 15 Furthermore, RCTs may not be necessary or appropriate to answer many questions, with the famous parachute example, “Parachute use to prevent death and major trauma related to gravitational challenge,” illustrating this in a humorous way. 16 While RCTs are clearly valuable, other sources of evidence exist, and EBM does not equal RCT.
There are two types of observational studies: analytic and descriptive. Analytic observational studies are case control and cohort studies. Descriptive observational studies are case series, case reports, and expert opinions. Generally speaking, observational studies are good for identifying associations, but are limited by less strict control of extraneous factors. Observational studies are intrinsically more at risk for bias and confounding. Bias is the extent to which a statistic actually represents the population parameter it is estimating. Confounding is the presence of an intermediate effect: the association between an event and effect is real, but acts through or with another event.
In a cohort study, a group of subjects, selected to represent the population of interest, is studied over time. 17 Subjects are disease free at the outset of the study. At distinct points in time data are collected about outcomes of interest and exposure to risk factors. Cohort studies may be prospective or retrospective. In prospective cohort studies the study is carried out from the present into the future. These studies may take a long time and be expensive as one waits for the outcome of interest to occur. Retrospective cohort studies look from a time point in the past to the present, and are limited by reliance on subject memory or data recording.
Case control studies are those studies in which subjects are enrolled based on whether or not they have an outcome or disease of interest. Exposure to the risk factor of interest is then compared between the subjects with the case, and those without, the controls. These studies are beneficial for being relatively quick, and for being able to study multiple risk factors of interest at once. They are also good studies for rare diseases. These are great studies for an exposure that is common, and also when an answer is needed quickly. For example, if a small number of cases of a severe allergic reaction are reported in patients who were all exposed to a new type of facial filler, a case control study would be a good first start for a quick answer. An obvious disadvantage to this type of study is that multiple diseases or outcomes of interest cannot be studied at once.
Case series, though providing a lower level of evidence, can be useful for generating hypotheses about associations. These hypotheses can then be studied with higher level research designs as appropriate to further study causality. The primary limitation to this type of study is the lack of a comparison group. A good case series will include the following elements: (1) a clear question, (2) clear inclusion/exclusion criteria, (3) a defined time period, (4) valid and reliable outcomes, (5) clear methodology, and (6) prospective evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree