Histopathology.
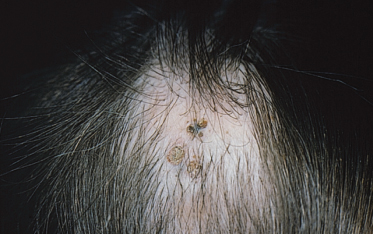
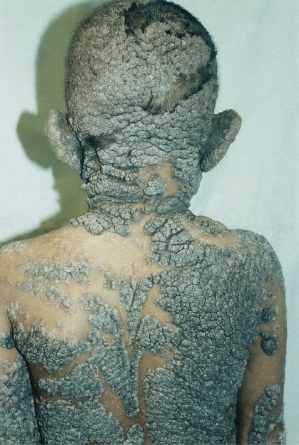
The histology of these naevi varies with the patient’s age. While the sebaceous component may be prominent in the neonatal period as a result of androgenic stimulation, they often regress and appear relatively undeveloped in childhood, when they may exhibit cords of undifferentiated cells similar to embryonic hair follicles. With the onset of puberty, glandular enlargement and epidermal proliferation often occur in addition to proliferation and hyperkeratosis of the epidermis as well as papillomatosis. In histological sections, large mature sebaceous glands abnormally high in the dermis, effectively replacing hair follicles, are noted, some of which empty directly to the skin surface. Ectopic apocrine glands may be present as well as persistence of the primordial follicular structures.
Neoplasia.
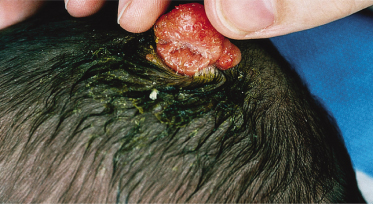
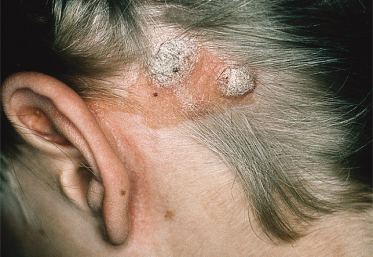
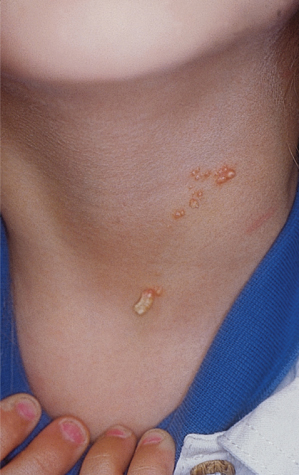
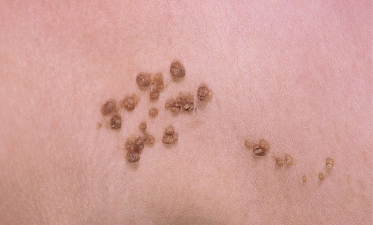
An increased rate of development of secondary tumours within sebaceous naevi has been long recognized. The most common secondary neoplasms are syringocystadenoma papilliferum (Fig. 110.7) and trichoblastomas (areas of basaloid hyperplasia often mimicking basal cell carcinoma, but which behave in a benign fashion). True basal cell carcinomas may occur in these lesions, although their frequency is debated, they appear exceedingly rare before puberty. A large retrospective study of 596 sebaceous naevi noted an incidence of basal cell carcinoma of 0.8% and benign tumours in 13.6% with syringocystadenoma papilliferum and trichoblastoma (differentiated from basal cell carcinoma via pattern analysis) as the most common. In this study viral warts were rare [7]. The incidence of secondary neoplasia increases with age, with the vast majority of secondary tumours appearing beyond the age of 20 years.
An increased incidence of viral warts has been noted in multiple reports and one recent study using DNA from formalin-fixed specimens detected human papilloma virus (HPV) DNA in the majority (82%) of NS tested. The majority of the HPV types were with genitomucosal or epidermodysplasia verruciformis associated and the most common subtype, HPV 16, appeared to exhibit genomic integration [8]. Further studies with fresh tissue and appropriate controls need to be performed to validate this result. It is unclear if the presence of HPV reflects secondary infection of already abnormal naevoid skin or is in fact primary.
Management.
Management of these lesions is controversial. Various ablative treatments have been reported, including cryotherapy, electrodessication, ablative laser treatments (CO2) and photodynamic therapy [9]. All ablative options carry the risk of leaving residual naevus that could recur or generate neoplasia. Thus, the definitive treatment, if warranted, remains surgical removal. The need for and timing of surgical removal of NS is controversial and depends on many factors [7,9–11]. Excision of NS in the first years of life is often technically less complicated although it carries a slightly higher risk of later development of new or residual lesions peripheral to the excision margin as a result of lack of visibility of the naevus during infancy. Removal prior to thickening at puberty is often advocated but depends on the wishes of the patient, their family, the size and location of the lesion as well as the expected scar [12,13].
Many NS will be managed solely by observation. Patients and their families should be counselled on how to observe these lesions and new growths biopsied and removed as appropriate.
Associated Syndromes.
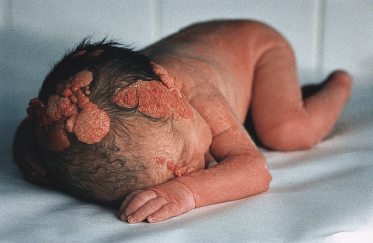
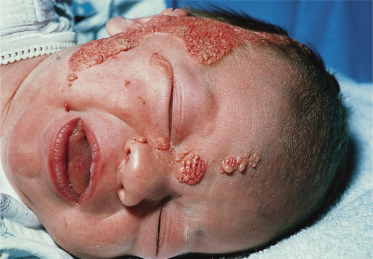
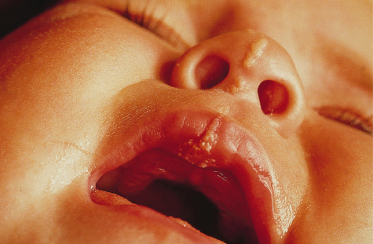
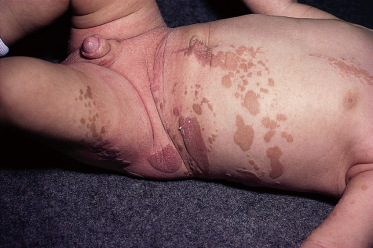
Syndromes associated with NS include naevus sebaceous syndrome (sebaceous naevus syndrome and Schimmelpenning–Feuerstein–Mims syndrome). Extracutaneous abnormalities associated with NS include CNS, skeletal and ocular abnormalities. CNS changes most often manifest clinically as mental retardation, seizures and hemiparesis, and occurred in approximately 7% of patients in one report [5]. The incidence of associated abnormalities appears to increase with increasing size of the naevus and in those located on the central face. In the above report, approximately 79% of affected patients exhibited mental retardation and 57% had seizure activity. Despite this, neuroimaging was normal in 75% of the group. Abnormalities detected in patients with sebaceous naevus syndrome include enlarged ventricles, intracerebral calcification, heterotopic grey matter, gyral malformation, cortical hypoplasia, hemimegalencephaly, as well as intracranial tumours and arachnoid cysts, typically ipsilateral to the naevus.
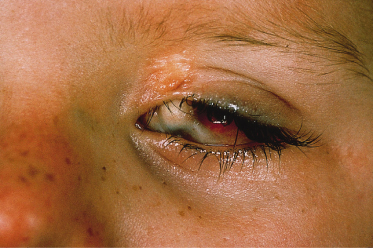
Ocular lesions reported in association with NS include choristomas (typically, complex and epibulbar, occasionally bilateral) and colobomas (eyelid colobomas are the most frequent association) (Fig. 110.8). Naevus sebaceous may be the EN most commonly associated with ocular lesions. In the Rogers et al. [14] report, 9% of patients with EN had ocular abnormalities, with 6.7% having strabismus. In cases of EN syndromes with extracutaneous findings, ocular lesions were present in 39%, with lipodermoids the most common (20%) followed by colobomas (9%).
Fig. 110.8 Repaired coloboma of the upper eyelid and complex ocular choristoma in a patient with the sebaceous naevus syndrome.
Skeletal lesions were present in 15% of the Rogers et al. [14] series although many of the findings were minor. In a large series of EN syndromes, 50% exhibited skeletal abnormalities. While various lesions were reported, many exhibited an overall overgrowth phenotype [15].
A recent report describes a neonate exhibiting a sebaceous naevus of the scalp, CNS malformations, membranous aplasia cutis congenita, limbal dermoid and giant congenital melanocytic naevus (contiguous with the NS) with associated neurocutaneous melanosis. The authors termed this constellation of findings ‘SCALP syndrome’ [16]. As a solitary case it remains to be seen whether this is a true syndrome or a chance association.
Didymosis aplasticosebacea is a recently described entity consisting of associated aplasia cutis congenita (often multiple) and NS. Affected patients often exhibit ocular lesions as above. The authors suggest that twin spotting (didymosis) represents a genetic mechanism by which these defects could arise. As causative genes for either condition are currently unknown this awaits molecular evaluation [17].
Follicular Naevus
Follicular naevus is also known as naevus comedonicus and comedo naevus. A review of the literature suggests that there is heterogeneity within this particular class of EN, which in general exhibits differentiation towards hair follicles. It is likely that several different entities have been reported under this moniker in the past and more refined histologic and molecular investigations are needed to clarify this EN subset.
Pathogenesis.
No genetic cause for naevus comedonicus has been elucidated. These naevi appear to demonstrate differentiation towards follicular structures and some exhibit diminutive hair shafts and sebaceous lobules at the base of the lesions. There has been one report of an extensive naevus comedonicus occurring in a case of Alagille syndrome [18].
One report has described mosaicism for mutations in FGFR2 in a patient with a linear acneiform naevus. However, the clinical appearance of the lesion in this report suggests a Blaschkolinear area of intensified acne caused by the underlying FGFR2 mutation rather than a classic naevus comedonicus [19].
Clinical Features.
These lesions may be limited or extensive and can occur on the head, neck, trunk and limbs. They appear as grouped or linear dilated follicles or comedones, often filled with keratinaceous débris (Fig. 110.9). If the keratinaceous plugs are removed the remaining lesions have a pitted appearance. While typically present at birth these naevi may develop during adolescence. After puberty, these naevi may develop deeper nodulocystic lesions which can resolve with scarring. Importantly, segmental basaloid follicular hamartomas may clinically resemble naevus comedonicus [20].
Histopathology.
Naevus comedonicus exhibit deep wide epidermal invaginations derived from follicular structures, which contain keratin. Small sebaceous glands may be seen opening into the lower portion of the structure. Hair is rarely formed. The epidermis between comedones may exhibit hyperkeratosis, papillomatosis and acanthosis typical of non-organoid EN. Some lesions resemble a collection of dilated pores of Winer.
Neoplasia.
Tumours reported in these naevi include those of follicular origin such as trichilemmal cysts, apocrine tumours and basal cell carcinomas. It is likely that basal cell carcinomas are rare in these naevi and that previously associated cases may represent misdiagnosis of segmental basaloid follicular hamartomas or other alternative differential diagnoses [21].
Management.
The definitive treatment is excision but this may not be feasible for large lesions. Treatment of actively infected lesions may be necessary and long-term treatment with oral antibiotics has shown benefit for deeper inflammatory lesions. Topical and oral retinoids have exhibited little sustained benefit. Favourable responses to daily application of 12% ammonium lactate have been reported [22,23].
Associated Syndromes.
The most common extracutaneous abnormality associated with naevus comedonicus is ipsilateral cataract [21]. Additional reported extracutaneous findings include corneal changes, electroencephalogram (EEG) abnormalities, CNS abnormalities, depigmented hairs and skeletal abnormalities including hemivertebrae, fused vertebrae, occult spina bifida, supernumerary digit, syndactyly, absent fifth ray of the hand, limb hypoplasia and scoliosis.
Additional Forms of Reported Organoid Epidermal Naevi
Trichilemmal Cyst Naevus/Naevus Trichilemmocysticus
A ‘trichilemmal cyst naevus’, also termed naevus trichilemmocysticus, has been described in a middle-aged woman who exhibited hyperkeratotic skin lesions from birth. Filiform hyperkeratosis arose in the first years of life followed within another few years by the development of cystic lesions within the Blaschkolinear distribution of the naevus. The patient also later developed juvenile idiopathic arthritis and osteomalacia from dietary and lifestyle-induced vitamin D deficiency, which led to spontaneous fractures. The keratotic areas exhibited clinical and histological similarity to porokeratotic eccrine naevi while the cysts were trichilemmal on histology. At puberty the cysts enlarged, with some draining and some becoming inflamed. This patient exhibited overlap between several EN including naevus comedonicus, porokeratotic eccrine naevus, linear basaloid follicular hamartoma, acneiform naevus and naevus corniculatus [24]. A recent report describes a child with a clinically identical lesion, strengthening the delineation of this as a unique entity [25].
Nevoid Basaloid Follicular Hamartomas
A syndrome of segmentally arranged basaloid follicular hamartomas has been proposed by Happle based on a series of previously described patients in the literature. These patients exhibit multiple basaloid follicular hamartomas following Blaschko’s lines. These lesions may exhibit a central umbilication similar to a comedo leading to the erroneous clinical diagnosis of naevus comedonicus. The involved area may exhibit hypopigmentation or hyperpigmentation as well as regions of atrophoderma. Hypertrichosis and hypotrichosis have also been reported. Palmar and/or plantar pitting has also been noted in some patients. Additional findings in this syndrome include skeletal anomalies such as cervical ribs, malformed thumbs, polydactyly, overgrowth or undergrowth of the long bones, saddle or socket nose deformity and scoliosis. A variety of dental anomalies have been described including anodontia and hypodontia. Cerebral defects associated with the syndrome include mental retardation and deficiency as well as gait anomalies. Previously described patients have not exhibited mutations in the Patched (PTCH) gene as would be typical of naevoid basal cell carcinoma syndrome [20]; however, testing of lesional keratinocytes would be necessary to confirm this.
Eccrine Naevi/Mucinous Eccrine Naevi
Overproliferation of eccrine glands in a naevoid manner has been described rarely in the literature. Some, in addition to an overabundance of eccrine glands, have exhibited excess mucin production histologically, and have been termed mucinous eccrine naevi. Such lesions have been reported to exhibit a variety of clinical forms, from papules, erythematous nodules, brownish and irregular nodules, well-defined erythematous swollen patches, triangular well-circumscribed areas of brown hyperpigmentation to localized brown plaques. Lesions have been located on the lower extremities, buttock and toes. Many have exhibited hyperhidrosis [26].
Mucinous Naevi
A rare entity, termed mucinous naevus, is described as exhibiting two clinical/histopathologic types:
1 Connective tissue naevus of the proteoglycan type (CNTP); and
2 Combined epidermal and CNTP.
Such lesions often follow a linear distribution and are composed of flesh-coloured to brownish papules or plaques. Some resemble keratinocytic EN clinically but, in addition to the typical histologic findings of keratinocytic EN, exhibit characteristic and diffuse mucin deposition in the superficial dermis. Recently, the mucin-producing cells have been identified as CD34+ fibroblasts [27]. Many lesions are congenital although acquired lesions are described. Spontaneous regression of these lesions has not been reported.
References
1 Rogers M. Epidermal nevi and the epidermal nevus syndromes: a review of 233 cases. Pediatr Dermatol 1992;9:342–4.
2 Hafner C, van Oers JM, Vogt T et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest 2006;116:2201–7.
3 Hafner C, Lopez-Knowles E, Luis NM et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Acad Sci U S A 2007;104:13450–4.
4 Honeycutt KA, Koster MI, Roop DR. Genes involved in stem cell fate decisions and commitment to differentiation play a role in skin disease. J Invest Dermatol Symp Proc 2004;9:261–8.
5 Davies D, Rogers M. Review of neurological manifestations in 196 patients with sebaceous naevi. Australas J Dermatol 2002;43:20–3.
6 Correale D, Ringpfeil F, Rogers M. Large, papillomatous, pedunculated nevus sebaceus: a new phenotype. Pediatr Dermatol 2008;25:355–8.
7 Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol 2000;42:263–8.
8 Carlson JA, Cribier B, Nuovo G, Rohwedder A. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol 2008;59:279–94.
9 Dierickx CC, Goldenhersh M, Dwyer P et al. Photodynamic therapy for nevus sebaceus with topical delta-aminolevulinic acid. Arch Dermatol 1999;135:637–40.
10 Orchard DC, Weston WL, Morelli JG. Tumors arising in nevus sebaceus. J Am Acad Dermatol 2001;45:793–4.
11 Barkham MC, White N, Brundler MA, Richard B, Moss C. Should naevus sebaceus be excised prophylactically? A clinical audit. J Plast Reconstr Aesthet Surg 2007;60:1269–70.
12 Santibanez-Gallerani A, Marshall D, Duarte AM, Melnick SJ, Thaller S. Should nevus sebaceus of Jadassohn in children be excised? A study of 757 cases, and literature review. J Craniofac Surg 2003;14:658–60.
13 Rosen H, Schmidt B, Lam HP, Meara JG, Labow BI. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol 2009;26:676–81.
14 Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol 1989;20:476–88.
15 Grebe TA, Rimsza ME, Richter SF, Hansen RC, Hoyme HE. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet 1993;47:24–30.
16 Lam J, Dohil MA, Eichenfield LF, Cunningham BB. SCALP syndrome: sebaceous nevus syndrome, CNS malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus (giant congenital melanocytic nevus) with neurocutaneous melanosis – a distinct syndromic entity. J Am Acad Dermatol 2008;58:884–8.
17 Demerdjieva Z, Kavaklieva S, Tsankov N. Epidermal nevus syndrome and didymosis aplasticosebacea. Pediatr Dermatol 2007;24:514–6.
18 Woods KA, Larcher VF, Harper JI. Extensive naevus comedonicus in a child with Alagille syndrome. Clin Exp Dermatol 1994;19:163–4.
19 Munro CS, Wilkie AO. Epidermal mosaicism producing localised acne: somatic mutation in FGFR2. Lancet 1998;352:704–5.
20 Happle R, Tinschert S. Segmentally arranged basaloid follicular hamartomas with osseous, dental and cerebral anomalies: a distinct syndrome. Acta Derm Venereol 2008;88:382–7.
21 Lefkowitz A, Schwartz RA, Lambert WC. Nevus comedonicus. Dermatology 1999;199:204–7.
22 Bordel MT, Miranda A. [Unilateral nevus comedonicus: efficacy after treatment with 12% ammonium lactate.] Actas Dermosifiliogr 2006;97:150.
23 Milton GP, DiGiovanna JJ, Peck GL. Treatment of nevus comedonicus with ammonium lactate lotion. J Am Acad Dermatol 1989;20:324–8.
24 Tantcheva-Poor I, Reinhold K, Krieg T, Happle R. Trichilemmal cyst nevus: a new complex organoid epidermal nevus. J Am Acad Dermatol 2007;57(5 Suppl):72–77.
25 Lang SC, Bauer B, Brocker EB, Hamm H. Naevus trichilemmocysticus: the first paediatric case of a newly delineated organoid naevus. J Eur Acad Dermatol Venereol 2010;Apr 5(epub ahead of publication).
26 Chen CW, Tsai TF, Chen YF, Hung CM. Congenital mucinous eccrine nevus in a 5-month-old girl with frequent intertriginous dermatitis. Pediatr Dermatol 2008;25:573–4.
27 Tardio JC, Granados R. The cellular component of the mucinous nevus consists of CD34-positive fibroblasts. J Cutan Pathol 2010;37:1019–20.
Non-Organoid Epidermal Naevi
Keratinocytic Epidermal Naevi
Along with NS, keratinocytic EN (composed mostly of acanthotic epidermis without excess sebaceous glands or other appendageal structures) are the most common type of EN.
Pathogenesis.
Activating germline mutations in fibroblast growth factor receptor 3 (FGFR3) are known to cause autosomal dominant achondroplasia and thanatophoric dysplasia, both of which are associated with acanthosis nigricans of skin. Given the similarity between keratinocytic EN and acanthosis nigricans, specimens of such EN were screened for similar mutations. The authors found activating FGFR3 mutations in 33% of examined specimens, most of which were the same mutation, R248C. In four samples where adjacent normal skin could be examined no mutations were noted. In specimens where multiple samples were available from the same lesion, all showed the same mutation, suggesting clonal derivation of the naevoid tissue and validating the underlying mosaicism. These activating FGFR3 mutations have also been detected in seborrhoeic keratosis; and several forms of human cancer including urothelial carcinoma (also noted in some patients with widespread EN); cervical and colorectal carcinoma and multiple myeloma. The most common activating mutation (R248C) in FGFR3 results in unpaired cysteine amino acid residues in crucial regions of the FGFR3, allowing disulphide bond formation between two receptors with resultant homodimerization and ligand-independent receptor activation. Other mutations lead to the formation of hydrogen bonds between two receptors resulting in the same effect. The authors also investigated multiple NS and found no such mutations in those lesions [1].
Given the association of FGFR3 mutations with PIK3CA mutations in bladder carcinoma, EN were also screened for common oncogenic PIK3CA mutations. PIK3CA mutations (specifically the E545G substitution, which is rare in cancers) were found in 27% of EN, 7/33 of which were not associated with FGFR3 mutations and 2/33 were. In total 14/33 (42%) of EN had either FGFR3 or PIK3CA mutations (also 42% of seborrheic keratoses). Five per cent of the lesions (EN and seborrheic keratosis) had both [2].
Clinical Features.
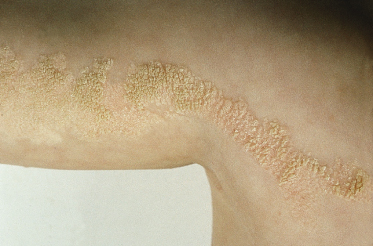
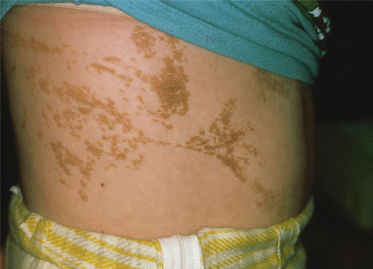
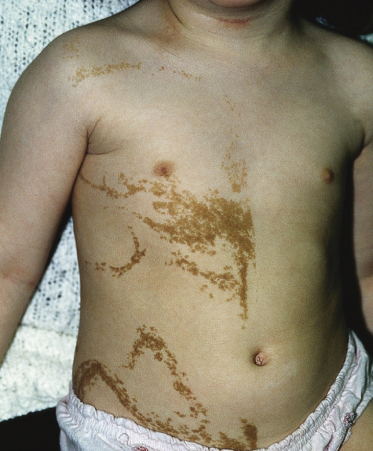
These lesions may vary from skin-coloured or pink to hyperpigmented and typically follow Blaschko’s lines; however, small focal lesions are reported (Figs 110.10–110.15). While often congenital, over half of keratinocytic EN appear after birth, typically in infancy, although lesions appearing as late as adolescence are reported. The lesions may extend over the first years of life. With time flat lesions may elevate. Acral lesions may be more warty in appearance and matrix involvement may lead to nail dystrophy. Body fold lesions may be softer. Some lesions can exhibit striking hyperkeratosis leading to an impressive verrucous appearance. Various older names for such keratinocytic EN reflect the wide variety of potential sizes and appearances, such as unilateral lesions (naevus unius lateris) or systemic lesions (ichthyosis hystrix or systematized EN). This form of EN appears to be less common on the head and neck.
Fig. 110.15 Keratinocytic naevus on the trunk, comprising multiple curved lines and demonstrating a midline cut-off.
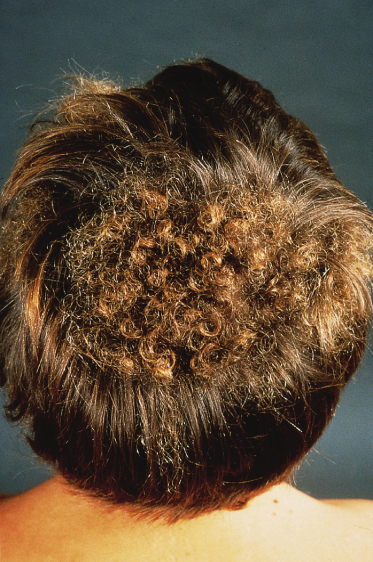
Patients exhibiting distinct patches of tightly curled hair localized to particular areas of the scalp are described and these lesions have been termed woolly hair naevi (Fig. 110.16). The lesional hair may be finer and lighter than the normal hair in addition to its increased curling. Woolly hair naevi may present in three clinical settings: as an isolated woolly hair naevus, in association with keratinocytic EN or an acquired form occurring in adulthood (late teens–early twenties) termed acquired progressive kinking of the hair [3]. In cases associated with EN the woolly hair naevus is typically ipsilateral but not contiguous with the EN although rare reports of one overlying the other exist [4,5]. The coexistence of woolly hair naevus, EN and oral white sponge naevus in a patient has been described [6]. Recent insights into the molecular basis of woolly hair in humans, including the discovery of keratin 74 mutations in autosomal dominant woolly hair syndrome have provided targets for future molecular investigation in such patients [7].
Histopathology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree