The severity between cases tends to differ and may change with time. Some patients state that as little as 10 min of direct sunlight between early spring and late autumn is all that is required to induce their eruption. In others, episodes are mild and may only occur once yearly during a summer holiday in a hot climate. For many, ultraviolet A (UVA; 290–320 nm) penetrating through window glass and even light cotton clothing can be responsible, producing problems at school, which may necessitate a move away from window seats. Nor is the season confined to spring/summer days – winter sunlight, particularly at high altitude when the snow is on the ground, can result in provocation.
A useful clinical feature is the frequently reported sparing of some maximally sunlight-exposed areas. Patients often comment that, surprisingly, their face and hands, areas normally exposed, are not involved. This is thought to relate to the hardening process seen as the summer progresses. This sparing phenomenon is not unique to PLE and is seen in some patients with actinic prurigo (AP) and solar urticaria (SU).
When presented with a clinically affected patient who is either too young to describe or is uncertain of the role of light, careful examination of areas of maximal sun-exposed skin is valuable. Sparing of the hair-bearing scalp, retro-auricular, precubital infranasal regions, inner aspect of arms and legs with involvement of the ‘V’ of the neck, outer aspects of the arms with a marked clothing cut-off usually helps with the diagnosis, although the clinician should be aware of ‘shadow site’ involvement in sunbathers exposed to reflected sunlight or in skiers, in whom normal shadow areas may be involved. Scarring is not associated with PLE, although excoriation may have this consequence.
In addition to direct sunlight, induction by artificial UV sources, e.g. tanning parlours, is increasingly common [15]. The importance of these UVA wavelengths is underlined by the fact that light transmitted by car glass is described as a causal factor in up to 49% of patients [10], with drivers and passengers reporting sunlight-induced unilateral PLE on those areas of skin adjacent to the side window. While the UVA wavelengths appear to be particularly effective at provoking PLE, UVB wavelengths are also capable of induction in the majority of patients [16,17].
Prognosis.
Spontaneous resolution has been reported, although only in a minority [18]. The condition seems to persist in most patients, although it may vary in its severity.
Differential diagnosis.
Although lupus erythematosus (LE) is usually easily distinguished, there are occasions when the skin lesions of LE may be clinically and histologically similar to PLE. It is important to assess the circulating (antinuclear antibody) ANA as well as anti-SSA (Ro) and anti-SSB (La) levels. Contact and photocontact allergy to sun screens may occasionally cause problems, although a dermatitic morphology should alert the clinician to these possibilities.
More frequently, difficulty can be encountered with those other members of the idiopathic photodermatoses group, SU and AP. The former can be excluded by phototesting. Hydroa vacciniforme (HV), at an early stage, may be confused, although the size of blisters and typical crusting with scar formation are characteristic. Patients frequently also initially describe their PLE as a heat rash, although the involved sites usually clarify the diagnosis. Lymphocytoma cutis and Jessner’s lymphocytic infiltration can both be confused with PLE. The chronic duration and characteristic clinical features of these conditions along with their histology will resolve the problem.
On occasions, early psoriasis at sites of previous PLE [19] or drug phototoxicity may cause confusion. The history and evolution of morphology are usually of diagnostic help.
Investigation.
If there is diagnostic doubt, in particular if SU is suspected, phototesting to exclude urticaria is advisable. Provocation of PLE lesions can be difficult and is best conducted in a specialized unit. Repeat challenge with broad-spectrum UVA/B can provoke the lesions in up to 80% of cases [20].
Therapy.
If the eruption does emerge, potent topical steroid use may provide some symptomatic relief, during which time the patients should avoid sunlight.
For the majority who are mildly affected, control of sunlight exposure coupled with the wearing of a topical high-factor broad-spectrum sunscreen will prevent PLE [21]. In others, a gradual sunlight exposure early in spring and summer will allow natural pigmentation and skin thickening to develop, allowing relative freedom over a holiday and for the rest of the summer. However, such controlled exposure may not be straightforward. For those more severely affected, the hardening process may be induced in a measured manner using artificial UV irradiation cubicles. Both UVB phototherapy and photochemotherapy (PUVA) have been assessed and found to be successful. In children, PUVA is contraindicated and phototherapy with UVB preferred. Over the last two decades, narrow-band UVB phototherapy (TL-01) has been found effective [21,22]. Typically, treatment is given three times weekly for 5 weeks early in each spring; incremental steps are best individualized (10–20%), avoiding provocation of PLE if at all possible. Although this approach in the very young (<7 years) may not be possible, the older age group in general do well. During a course of treatment there is often no need to expose areas other than the arms, legs, face and neck. Patients are encouraged to wear their summer clothes during the desensitization course. In cases of extreme severity, immunosuppression with systemic steroids, ciclosporin or azathioprine has been tried [23].
References
1 Rasch C. Om et polymorft (erytematost, vesikulost og ekzematoidt) lysudslet. Hospitalstid 1900;43:478–80.
2 Jansen CT, Helander I. Cell-mediated immunity in chronic polymorphous light eruptions. Acta Derm Venereol (Stockh) 1976;56:121–5.
3 Moncada B, Gonzalez-Amaro R, Baranda ML et al. Immunopathology of polymorphous light eruption. J Am Acad Dermatol 1984;10:970–3.
4 Norris PG, Hawk JLM. Successful treatment of severe polymorphous light eruption with azathioprine. Arch Dermatol 1989;125:1377–9.
5 Norris PG, Morris J, McGibbon DM et al. Polymorphic light eruption: an immunopathological study of evolving lesions. Br J Dermatol 1989;120:173–83.
6 De Gruijl FR. UV-induced immunosuppression in the balance. Photochem Photobiol 2008;84:2–9.
7 Muhlbauer JE, Bhan AK, Harrist TJ et al. Papular polymorphic light eruption: an immunoperoxidase study using monoclonal antibodies. Br J Dermatol 1983;108:153–62.
8 Lever F, Schaumburg-Lever G (eds). Histopathology of the Skin. Philadelphia: JB Lippincott, 1990: 232–3.
9 Ros A-M, Wennersten G. Current aspects of polymorphous light eruptions in Sweden. Photodermatology 1986;3:298–302.
10 Frain-Bell W. Cutaneous Photobiology. New York: Oxford University Press, 1985.
11 Ferguson J. Polymorphic light eruption and actinic prurigo. Curr Prob Dermatol 1990;11:127–46.
12 Ros A-M. Solar purpura – an unusual manifestation of polymorphous light eruption. Photodermatology 1988;5:47–8.
13 Elpern DJ, Morison WL, Hood AF. Papulovesicular light eruption. A defined subset of polymorphous light eruption. Arch Dermatol 1988;118:73–6.
14 Dover JS, Hawk JLM. Polymorphic light eruption sine eruptione. Br J Dermatol 1988;118:73–6.
15 Larko T. Treatment of psoriasis with a new UVB lamp. Acta Derm Venereol 1989;69:691–6.
16 Miyamoto C. Polymorphous light eruption: successful reproduction of skin lesions, including papulovesicular light eruption, with ultraviolet B. Photodermatology 1989;6:69–79.
17 Bilsland D, George SA, Gibbs NK et al. A comparison of narrowband phototherapy (TL-01) and photochemotherapy (PUVA) for severe polymorphic light eruption. Br J Dermatol 1993;129:708–12.
18 Jansen CT, Karvonen J. Polymorphous light eruption. A 7-year follow-up evaluation of 114 patients. Arch Dermatol 1984;120:862–5.
19 Przybilla B, Heppeler M, Ruzicka T. Preventative effect of an E. coli filtrate (Colibiogen) in polymorphous light eruption. Br J Dermatol 1989;121:229–33.
20 Janssens AS, Pavel S, Ling T et al. Susceptibility to UV-A and UV-B provocation does not correlate with disease severity of polymorphic light eruption. Arch Dermatol 2007;143:599–604.
21 Bilsland D, Ferguson J. The management of polymorphic light eruption. J Dermatol Treat 1992;3:99–101.
22 Gambichler T, Breuckmann F, Boms S, Altmeyer P, Kreuter A. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol 2005;52:660–70.
23 Honigsmann H, Hijyo-Tomoka MT. Polymorphous light eruption, hydroa vacciniforme, and actinic prurigo. In: Lim HW, Honigsmann H, Hawk JLM (eds) Photodermatology. New York: Informa Healthcare, 2007: 148–67.
Actinic prurigo
Definition.
Actinic prurigo (AP) is a rare [1] inflammatory photodermatosis that usually arises in childhood. Although Caucasians are affected, it appears more common in some Native American populations.
History.
The term ‘actinic prurigo’ was first introduced by Lopez Gonzalez in 1961 to describe an idiopathic photodermatosis of Amerindians. It is likely that this is the same condition labeled by Hutchinson in 1878 as ‘summer prurigo’ [2]. Another term used in the literature is hereditary PLE (HPLE) [3], which causes confusion with PLE. The disease is also present in Japanese [4] and Indian [5] patients.
Aetiology and pathogenesis.
The mechanism of AP is unknown; like PLE, it is triggered by sunlight, although a number of distinct features separate the two entities. As in the other idiopathic photodermatoses, no chromophore has been identified. A recent finding of interest has been the HLA link. Initially, an association was described in Amerindians [6], with confirmation within a Caucasian population of a link with HLA-DR4 (DRB1-0407) [7]. It does seem that, although there is a strong association, there may be cases that are HLA-DR4 negative [8]. Gene linkage with a TNF-α promoter polymorphism has been suggested but not confirmed [9].
Clinical features.
Actinic prurigo is characteristically a perennial problem, with maximum severity during the spring and sunshine months. Unlike most PLE patients, who are convinced of the relationship with sunlight exposure, in AP there is often uncertainty, at least initially, of a causal relationship. Calnan & Meara [10] found sunlight to be the stated precipitating factor in only 46% of their group. With a perennial complaint in which the role of sunlight is frequently denied, it is understandable why the diagnosis may be missed, a situation further complicated by occasional patients who report that cold and wind are causal factors.
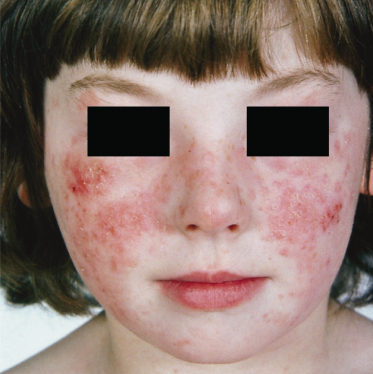
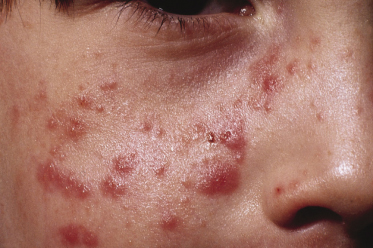
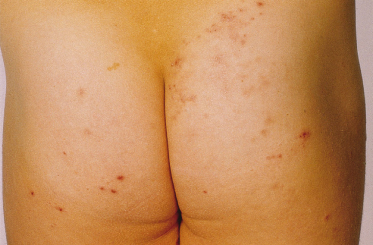
The skin response to sunlight has two phases. There is the several hours delayed, pruritic oedematous erythema often presenting as papules (Fig. 106.3) which may progress to vesiculation. Later, a chronic prurigo develops on both exposed and unexposed sites, often with nodules and plaques, which may become secondarily infected, leaving scars. All exposed sites are usually involved and it is uncommon to have the marked sparing of face and backs of hands commonly seen in PLE. As might be expected, patients only rarely describe hardening as the summer advances [11], although it is of interest that this does not exclude desensitization as a therapeutic approach. A characteristic finding of diagnostic value in AP is the involvement of covered sites (Fig. 106.4), the reasons for which are unclear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree