24 Nerve entrapment syndromes
Synopsis
 Diagnosis of nerve compression syndromes should be based on a thorough clinical evaluation, including history of vocational and nonvocational activities.
Diagnosis of nerve compression syndromes should be based on a thorough clinical evaluation, including history of vocational and nonvocational activities.
 Electrodiagnostic testing should be used primarily to confirm the clinical diagnosis or rule out other pathology.
Electrodiagnostic testing should be used primarily to confirm the clinical diagnosis or rule out other pathology.
 Carpal tunnel syndrome (CTS) and cubital tunnel syndrome are among the most common clinical entities evaluated by the hand surgeon.
Carpal tunnel syndrome (CTS) and cubital tunnel syndrome are among the most common clinical entities evaluated by the hand surgeon.
 Carpal tunnel release and cubital tunnel release under local anesthesia alone are safe and time- and cost-effective, as well as convenient for patients.
Carpal tunnel release and cubital tunnel release under local anesthesia alone are safe and time- and cost-effective, as well as convenient for patients.
 Current evidence suggests that ulnar nerve transposition is no better than simple decompression alone in the treatment of cubital tunnel syndrome.
Current evidence suggests that ulnar nerve transposition is no better than simple decompression alone in the treatment of cubital tunnel syndrome.
 Uncommon nerve entrapment syndromes such as quadrilateral space syndrome and suprascapular nerve compression should be considered in the differential diagnosis of chronic shoulder pain.
Uncommon nerve entrapment syndromes such as quadrilateral space syndrome and suprascapular nerve compression should be considered in the differential diagnosis of chronic shoulder pain.
Introduction
• Chronic nerve entrapment syndromes in the upper extremity are common and their occurrence is only likely to increase as risk factors such as diabetes, obesity, and advanced age become more prevalent in the general population.
• In most cases, the diagnosis can be readily made on the basis of history and physical examination.
• For more atypical presentations, consideration should be given to other neurologic conditions that mimic nerve entrapment syndromes such as mononeuritis, Parsonage–Turner syndrome, and motor neuropathies; not only will these conditions not respond to surgical decompression, they may in fact be exacerbated by operative management.
• When the diagnosis is unclear, electrodiagnostic tests and imaging can be of benefit.
• The purpose of the current chapter is to provide the reader with a comprehensive review of the pathophysiology, diagnosis, and treatment of nerve entrapment syndromes of the upper extremity.
Pathophysiology of chronic nerve compression
Given the associated morbidity of nerve biopsy in patients, most of our current understanding of the histopathologic changes associated with nerve entrapment syndromes stems from animal model studies. A consistent finding in these studies is that the severity of pathologic changes is dependent on the magnitude and duration of compression. An initial breakdown in the blood–nerve barrier is observed, followed by endoneurial edema.1 Along with perineural thickening, this edema may lead to the deposition of Renaut bodies within the substance of the nerve, further increasing endoneurial pressure. Increased endoneurial pressure disrupts the microneurial circulation, thereby inducing dynamic ischemia in the nerve.2
Clinically, these histopathologic changes correspond to paresthesias and an elevation in the static cutaneous sensory threshold as measured by Semmes–Weinstein monofilament testing. With increased compression, localized demyelination occurs followed by more diffuse demyelination.3 Demyelination is followed by remyelination. Schwann cells, stimulated by mechanical forces, proliferate and deposit thinner myelin.4 At this stage, patients will experience muscle weakness and will demonstrate increased pressure thresholds for quickly adapting fibers on vibrometry; two-point discrimination, measured in millimeters, remains unchanged. Superficial fascicles tend to undergo demyelination earlier and may explain varying patient symptoms within a single nerve distribution. In early CTS, for example, patients are more likely first to experience paresthesias in the long and ring fingers as the fascicles to these digits are most superficial within the median nerve. Unlike acute crush injuries, Wallerian degeneration is not seen until chronic nerve entrapment is particularly advanced.5,6 Only at this stage are anesthesia and muscle atrophy observed.
The double-crush phenomenon is an important concept in chronic nerve entrapment. First proposed by Upton and McComas, the double-crush concept holds that compression of a peripheral nerve at one site increases susceptibility to compression at another site along the same nerve.7 This phenomenon appears to be the result of the disruption of anterograde or, in the case of reverse double crush, retrograde axoplasmic flow of neurotrophic factors.8 The double-crush phenomenon has been demonstrated experimentally in animal models of chronic compression.9–12 Clinically, numerous examples of multiple sites of compression along the same nerve have been described and include reports of cervical disk disease and median nerve compression, CTS and pronator syndrome, and cubital tunnel syndrome and ulnar nerve compression in Guyon’s canal.13–16 The double crush concept suggests that two simultaneous points of compression, each of which might be insufficient to cause symptoms on its own, could together produce symptoms. In this way, decompression of one of the two sites might be sufficient to address a patient’s symptoms. The most distal nerve compression is typically decompressed first as it is usually the site of the more severe compression and lower surgical risk. If decompression in the distal extremity fails to relieve symptoms, decompression of the more proximal site, such as the intervertebral space or brachial plexus, should be considered.
Electrodiagnostic studies
Depending on systemic factors as well as the location and degree of compression, patients with nerve entrapment syndromes can present with a constellation of symptoms. Given the wide variability in symptoms, numerous attempts have been made at creating standardized diagnostic approaches to these conditions. For example, consensus groups have tried to establish a definitive list of symptoms for the diagnosis of CTS.17,18 Provocative tests such as Phalen’s, Tinel’s, and two-point discrimination tests might also be helpful in diagnosing CTS and other compression neuropathies; however, the positive predictive value of such tests alone is low.19–21 When an atypical presentation or comorbidities cloud the clinical picture, symptom criteria and provocative tests can be combined with electrodiagnostic studies to make the diagnosis.
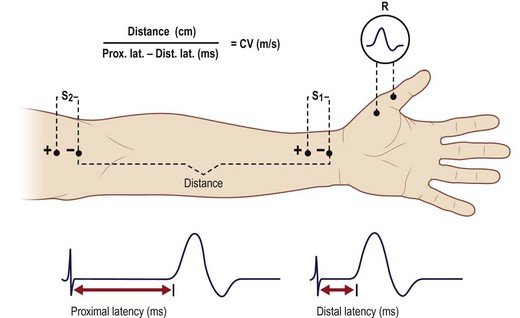
Two aspects of electrodiagnostic tests are most often used to diagnose nerve entrapment syndromes: nerve conduction studies (NCS) and electromyography (EMG). Of the two studies, the former are especially helpful. An NCS is performed by placing two electrodes on the skin, along the course of the nerve (Fig. 24.1). The first electrode is used to stimulate a peripheral nerve to fire, and the second electrode is used to record the characteristics of the generated action potential at some distance away from the stimulation point. The electrodes typically detect only larger, faster-conducting fibers. NCS provide a number of helpful measurements. Amplitude represents the size of the response and is roughly proportional to the number of depolarizing axons in the nerve. Latency is the delay in response following stimulation. Conduction velocity is determined by dividing inter-electrode distance by the latency.
For sensory nerves, recording electrodes are typically placed proximally along the nerve, toward the spinal cord, yielding a sensory nerve action potential (SNAP). For mixed motor-sensory and pure motor nerves, the recording electrodes are placed distally along the nerve toward or at the target muscle, providing a compound nerve action potential or compound motor action potential (CMAP), respectively.22,23 Another useful parameter elicited by NCS is the F wave. F waves are produced by antidromic conduction of the artificially stimulated action potential back to the anterior horn cells, which then discharge and send impulses back down the nerve following the CMAP. Decreased F-wave latencies suggest more proximal lesions within the nerve, such as plexopathies and radiculopathies, which may contribute to a double crush.24
In the early stages of chronic nerve compression, an increase in latency and a decrease in nerve conduction velocity are observed as focal demyelination occurs.22,23,25 CMAPs and SNAPs triggered distal to the compression site exhibit little to no decrease early in nerve entrapment syndromes. Over time, however, axonal damage occurs at the compression site, resulting in decreases first in SNAPs and later in CMAPs. As further axonal loss takes place, EMG studies demonstrate increased insertional activity, fibrillation potentials, positive sharp waves, or fasciculations. Axonal sprouting produces collateral reinnervation, the hallmark of which are short, high-amplitude “giant” MUPs on EMG. Following decompression, remyelination occurs and nerve conduction velocities typically return to normal with loss of “giant” MUPs.
Compression of the median nerve
Carpal tunnel syndrome
CTS is the most common nerve entrapment syndrome in the upper extremity, with an estimated incidence in the US of 1–3 cases per 1000 subjects per year and a prevalence of 50 cases per 1000 subjects per year.26 The economic burden from lost wages and productivity and treatment costs is considerable.27 Despite the prevalence of CTS, debate remains over the accurate diagnosis and optimal treatment of the condition.
Anatomy
The anatomic boundaries of the carpal tunnel are defined dorsally by the carpus, and volarly by the transverse carpal ligament (TCL), which spans from the hamate and triquetrum on the ulnar side to the scaphoid and trapezium on the radial side. The median nerve and flexor tendons (flexor pollicis longus (FPL), four flexor digitorum superficialis (FDS) and four flexor digitorum profundus (FDP) tendons) pass through the carpal tunnel. The median nerve is the most superficial structure within the canal. The median nerve may divide in the forearm or split within the carpal tunnel. Both presentations are associated with a persistent median artery. The recurrent motor branch to the thenar muscles usually arises off the median nerve in an extraligamentous position distal to the TCL (46–90%) (Fig. 24.2). Less frequently, the motor branch originates from beneath the TCL (subligamentous, 31%) or perforates the TCL (transligamentous, 23%).28 The palmar cutaneous branch typically arises off the radial aspect of the median nerve 5–6 cm proximal to the distal wrist crease. Additional branching pattern variations of the recurrent motor branch and palmar cutaneous branch have been described.29
Etiology
In the vast majority of CTS cases an underlying etiology cannot be identified. Women are more often affected than men.30 On histologic examination of idiopathic CTS, tenosynovial tissue demonstrates increased edema and fibrous thickening with minimal inflammation. Compression tends to be greatest at a point 1 cm distal to the distal wrist crease where the TCL is thickest.31 In rare instances, structural causes such as a persistent median artery, ganglion cyst, hemangioma, or proximal lumbrical origin can increase pressure within the carpal tunnel.
Systemic conditions, such as renal failure, thyroid disease, rheumatoid arthritis, and diabetes mellitus, may also predispose patients to CTS. CTS can occur in up to 45% of pregnant women during the third trimester but usually resolves postpartum.32 In the pediatric population, mucopolysaccharidoses are a frequent cause of CTS.33 To date, the only strong evidence linking occupation to CTS has been among operators of handheld vibratory tools.34 Scientific support for a causative role for other repetitive work activities (e.g., typing) in the development of CTS is otherwise lacking.35
Diagnosis/patient presentation
Threshold sensory tests such as Semmes–Weinstein monofilament measurements tend to be more sensitive for detecting early CTS than innervation density measurements.36 Manual testing of abductor pollicis brevis muscle strength as well as grip and pinch strength can also be helpful. Thenar atrophy has a high predictive value in CTS, but is rarely observed.37
Several provocative tests should be considered to aid in the evaluation of CTS. Tinel’s sign is elicited by gently percussing over the median nerve at the carpal tunnel. A positive sign is present if the patient describes an electrical shock sensation in the median nerve distribution. The low specificity and sensitivity of the sign may in part be due to the wide intra- and interexaminer variability with which it is elicited.38 Phalen’s test is performed by having the patient place the elbow on a table and flex the wrist for 60 seconds. The test is considered positive if the patient reports paresthesias in the median nerve distribution. Durkan’s median nerve compression test involves direct compression of the median nerve at the carpal tunnel for 30 seconds. A positive test is obtained if the patient reports numbness or tingling in at least one of the radial digits.39 Other less frequently used provocative tests include the reverse Phalen’s and tourniquet tests.40 For multiple reasons, including poor study design, variability in outcome measures and small datasets, no single test has been identified to diagnose CTS consistently. Of the above maneuvers, Durkan’s compression test performed with a calibrated pressure device demonstrates the highest sensitivity (89%) and specificity (96%).39 The sensitivities and specificities of Tinel’s sign and Phalen’s test are lower.19–21
Given the utility of patient symptoms and physical findings, attempts have been made to develop formal clinical criteria for the diagnosis of CTS. Graham et al. generated a list of six clinical criteria (CTS-6) for the diagnosis of CTS, based on a validated statistical analysis of recommendations by an expert panel. The six criteria included: (1) nocturnal numbness; (2) numbness and tingling in the median nerve distribution; (3) weakness and/or atrophy of the thenar muscles; (4) Tinel sign; (5) Phalen’s test; and (6) loss of two-point discrimination.41 Similarly, an American Academy of Orthopaedic Surgeons (AAOS) panel reviewed the available literature and developed recommendations for diagnosing CTS; high-level evidence was lacking to support the majority of recommendations.42
CTS remains a clinical diagnosis, and the role of electrodiagnostic and imaging studies remains complementary. Electrodiagnostic tests are routinely performed, yet their validity is largely unproven. These studies have been shown to be poor predictors of symptom severity or functional impairment.43 Patients with clinical evidence of CTS and negative NCS studies have been shown to have identical clinical improvement after surgery as patients with positive NCS findings.44 Electrodiagnostic tests are most useful in ruling out other pathology, monitoring treatment response in complicated cases, or making the diagnosis when the patient is a poor historian. Distal motor and sensory latencies greater than 4.5 and 3.5 ms, respectively, are generally considered positive.45 Routine radiographs have a low yield for underlying abnormalities.46 Ultrasound examination of the cross-sectional area of the median nerve shows some promise in predicting clinical symptoms while obviating the need for more invasive electrodiagnostic studies; however, for this diagnostic tool to be useful, validated normative data must first be established.47,48
Patient selection
Nonoperative treatment
As underscored by AAOS treatment recommendations, there is a role for both nonsurgical and surgical modalities in the management of CTS (Table 24.1).49 Splinting is the most widely used nonsurgical modality. On the basis of two well-designed studies, splinting has been shown to be more effective than no treatment at relieving symptoms for at least 3 months.50,51 No difference has been demonstrated between continuous splinting and nocturnal splinting alone.52
Table 24.1 American Academy of Orthopaedic Surgeons clinical practice guidelines for the treatment of carpal tunnel syndrome (CTS): recommendations
Steroid injection is another commonly used nonsurgical treatment that has been extensively reviewed.53 Steroid injection provides greater clinical improvement in symptoms 1 month after injection compared to placebo.54,55 In addition, local injection provides superior symptom relief compared to systemic steroid administration for up to 3 months.56 Two local corticosteroid injections do not provide significant added clinical benefit compared to one injection.57 Steroid injection in combination with splinting has also been shown to be better than splinting only at 6-month follow-up.58 Although systemic corticosteroids have been shown to provide clinical improvement, concerns remain over the risk of steroid-related complications.59
Ultrasound has also been shown to be an effective treatment.60 High-level evidence is lacking to support other nonsurgical modalities including iontophoresis, laser therapy, heat therapy, diuretics, vitamin B, and nonsteroidal anti-inflammatories (NSAIDs).
Treatment/surgical technique
The wide-awake approach (local infiltration without sedation or tourniquet) has been shown to be safe and cost-effective and is the preferred method of the authors.61,62 Tumescent field infiltration of 20 mL of 1% lidocaine with 1 : 100 000 epinephrine is performed (Fig. 24.3). A 3-cm incision is made in line with the flexed ring finger paralleling the thenar crease and ending just distal to the distal wrist flexion crease. The palmar fascia and TCL are incised longitudinally to expose the median nerve. In cases where a prominent palmaris brevis muscle is present, the senior author attempts to preserve it, dividing the TCL deep to it (Fig. 24.4). The ligament and forearm fascia releases are extended distally to divide all distal TCL fibers completely in the fat pad and proximally beneath the wrist flexion crease for about 1 cm into the forearm. Placement of the incision ulnar to the thenar skin crease avoids the palmar cutaneous branch, while direct visualization of the median nerve allows identification of an anomalous origin of the motor branch. The authors prefer to perform skin closure using buried intradermal 5-0 absorbable monofilament sutures, thereby obviating the need for suture removal (as shown in video 2, which also contains detailed advice provided to patients intraoperatively). A light dressing only is applied, as postoperative splinting has not been shown to improve pain relief or surgical outcome.63 Patients are allowed to remove the dressing and shower the day after surgery. A new dressing may be applied for padding and protection for up to a week. Patients are advised to elevate and rest the hand for the first two postoperative days. In most cases, ibuprofen and acetaminophen provide sufficient analgesia over this time. After the second postoperative day, any residual pain is usually the result of overexertion; a gradual return to full activities is advised with pain serving as a guide. Most patients can return to full work activities by 8 weeks. Patients are advised that mild discomfort at the operative site and decreased grip strength can persist for several more months.
Endoscopic carpal tunnel release (ECTR) techniques were developed in an attempt to avoid problems occasionally experienced following OCTR, namely scar tenderness and pillar pain. Popular approaches include the dual-portal technique of Chow and the single-portal technique of Agee.64,65
Both OCTR and ECTR are practiced widely, with proponents of both techniques continuing to debate the merits of one over the other. A common argument in favor of ECTR over OCTR has been reduced postoperative pain and a shorter return to vocational activities. Although these findings have been borne out in some studies,66,67 other studies show that any differences between techniques in patient symptoms, function, and satisfaction equalize by 1 year.68,69 Supporters of OCTR have in turn cited a higher incidence of postoperative neurovascular complications as a reason to avoid ECTR.70 More recent studies have failed to support this higher risk in ECTR.66–69,71 Cost has been another factor in the debate between OCTR and ECTR. A 1998 cost-effectiveness analysis comparing the two techniques concluded that ECTR is cost-effective provided major complications occur 1% less often than in OCTR.72 Previous prospective studies have not shown a substantial cost difference between the techniques if the cost of ECTR equipment is excluded.66–68
Outcomes and complications
Nonsurgical management has clearly been shown to provide short-term relief of CTS; however long-term studies of these modalities is lacking.53,57,59 Long-term outcome studies have focused on surgical treatment. Several studies have examined factors to predict response to carpal tunnel release. Burke et al. used a validated self-assessment tool to demonstrate that severity and not duration of symptoms preoperatively was the greatest predictor of degree of improvement.73 While advanced thenar wasting and complete anesthesia are not likely to resolve following surgery, pain associated with CTS typically does, regardless of its duration. Although response to carpal tunnel release is widely held to be worse among elderly and diabetic patients, this has not been borne out by a number of recent studies.74–76 Overall, outcomes following carpal tunnel release are excellent, with patient satisfaction, symptom relief, and functional improvement observed in more than 94% of cases.75,77
Complications of carpal tunnel release include, but are not limited to, injuries to the motor and palmar cutaneous branches and main trunk of the median nerve; hypertrophic scarring; pillar pain; superficial palmar arterial arch injury; incomplete TCL release; tendon adhesions; infection; wound hematoma; stiffness and recurrence. The most common complication with OCTR is pillar pain (25%), followed by laceration of the palmar cutaneous branch of the median nerve.78 Incomplete TCL release is the most frequent complication of ECTR.66 Recurrent CTS symptoms are seen in up to 20% of cases.79 Revision carpal tunnel surgery generally results in less favorable outcomes. Revision techniques described include complete division of the TCL, nerve grafting or neurolysis with fat transfer, muscle transfer or vein wrapping.79,80
Median nerve compression in the proximal arm and elbow
Diagnosis/patient presentation
Compression of the median nerve in the forearm is far less common than CTS. Once formed by the terminal divisions of the medial and lateral cords of the brachial plexus, the median nerve travels medial to the brachial artery, giving off no branches to muscles above the elbow. In the distal upper arm proximal and medial to the medial epicondyle, a supracondylar process, with its attached ligament of Struthers, can be found in over 1% of the population; as the median nerve travels deep to the ligament of Struthers it can be compressed (Fig. 24.5).81 As it enters the forearm, the median nerve can become compressed at several other sites proximal to the carpal tunnel, including the bicipital aponeurosis or lacertus fibrosis, anomalous muscles (such as Gantzer’s muscle, an accessory FPL muscle), and anomalous arteries. Most common of the median nerve entrapment syndromes in the forearm are pronator syndrome and anterior interosseous nerve (AIN) syndrome.
Pronator syndrome
Pronator syndrome is very uncommon when compared with CTS. It results from the compression of the median nerve as it passes between the two heads of the pronator teres or under the fibrous edge of the FDS arch. Patients typically present with aching pain in the proximal volar forearm with paresthesias extending into the median nerve distribution distally. These sensory symptoms can make discriminating between pronator syndrome and CTS challenging. In the latter condition, however, sensation in the palmar cutaneous nerve distribution is preserved as this nerve branch arises proximal to the carpal tunnel. Another helpful clinical maneuver is to have the patient attempt to pronate the neutral forearm against resistance; if symptoms are elicited during this maneuver as the elbow is extended, compression at the level of pronator teres should be suspected.82 If pain or paresthesias are triggered by resisted flexion of the fully supinated forearm, the lacertus fibrosus may represent the site of compression. Finally, if resisted contraction of the FDS to the long finger reproduces symptoms, the FDS fibrous arch is a more likely compression point. The examiner must take care during resisted tests not to elicit signs from unrelated pathology such as wrist osteoarthritis. Electrodiagnostic tests should be considered to exclude other sites of compression, but are not very helpful in the diagnosis of pronator syndrome itself.
Anterior interosseous nerve syndrome
Also known as Kiloh–Nevin syndrome, after the individuals who first fully described the condition, AIN syndrome results from the isolated compression of the AIN under the fibrous arch of the FDS or the pronator teres.83 Patients with AIN syndrome will describe weakness of pinch, which affects activities such as picking up small objects and writing, without sensory loss. There may be a history of forearm pain preceding the motor weakness. If instead there is a history of transient shoulder pain following a viral infection that precedes the motor weakness, the surgeon should consider the possibility of Parsonage–Turner syndrome (brachial neuritis). These two entities need to be distinguished as the management of AIN syndrome is directed toward the elbow and forearm. In a patient with rheumatoid arthritis, rupture of the FPL needs to be ruled out by ultrasound, magnetic resonance imaging (MRI), or exploration of a small portion of FPL under local anesthesia.
Because the AIN innervates the FPL, FDP to the index and middle fingers, and pronator quadratus, patients with a complete AIN palsy should exhibit no motor function in these three muscles unless a Martin–Gruber connection or ulnar contribution to the middle finger FDP is present.84 In incomplete AIN palsy, isolated FPL involvement is most typically observed. Severe weakness of FPL and the index finger FDP results in a characteristic inability to make an “OK” sign. More subtle weakness of these muscles can be demonstrated by having the patient hold a piece of paper between the thumb and index finger against resistance; with an AIN palsy, the patient will compensate by keeping the thumb interphalangeal and index distal interphalangeal joints extended. Electrodiagnostic studies may be helpful in ruling out more proximal lesions such as a brachial neuritis. MRI has been described but is not widely used in the diagnosis of AIN syndrome.85
Patient selection
Nonoperative treatment
Conservative management such as avoidance of aggravating activities, rest, and use of NSAIDs has been shown to be effective in both pronator and AIN syndromes. Up to 70% of patients diagnosed with pronator syndrome respond to nonsurgical treatment.86 When nonsurgical approaches fail, or when a clear anatomic cause such as a tumor is identified, surgical decompression of the median nerve in the forearm should be considered. Controversy remains over the appropriate period to continue conservative treatment before considering surgical intervention. Although most authors recommend surgical exploration after 12 weeks of persistent symptoms despite nonsurgical treatment, spontaneous recovery has been shown to occur as long as 1 year following symptom onset.87–89
Treatment/surgical technique
For both pronator and AIN syndromes, access to the median nerve in the forearm is obtained through a zigzag or lazy-S incision over the antecubital fossa. Decompression is begun proximal to the antecubital crease in order to identify the median nerve easily and explore for a ligament of Struthers. Decompression in the forearm includes release of the bicipital aponeurosis and the proximal edge of the FDS arch (Fig. 24.6). The superficial or humeral head of the pronator teres is then released fully or lengthened using a step cut. The arm can be immobilized for 1 week in a well-padded posterior splint with the elbow at 90° and the forearm in neutral.
Compression of the ulnar nerve
Ulnar nerve compression in Guyon’s canal
Anatomy
Ulnar nerve entrapment in Guyon’s canal, also known as ulnar tunnel syndrome, is very uncommon. Guyon’s canal is defined radially by the hamate, volarly by the volar carpal ligament, dorsally by the TCL, and ulnarly by the pisiform and flexor carpi ulnaris (FCU); more distally, nerve branches course deep to the hypothenar muscles (Fig. 24.7). Depending on the location of the compression with Guyon’s canal, symptoms may be a combination of motor and sensory (zone I), purely motor (zone II), or purely sensory (zone III).
Etiology
The most common cause of ulnar nerve compression at the wrist is external compression by a space-occupying lesion, most commonly a ganglion.90 The zone within which the ganglion arises can typically be determined on the basis of patient symptoms and clinical examination alone.91 Tumors such as giant cell tumor, neurilemmoma, and lipoma may also give rise to ulnar tunnel syndrome.92 Numerous cases of anomalous hypothenar muscles impinging on the ulnar nerve have been reported.93–95 Hypothenar hammer syndrome can be associated with compression of the ulnar nerve in Guyon’s, most commonly within zone III.96 Other less common causes of ulnar nerve compression at Guyon’s canal include rheumatoid arthritis and carpal fractures.97–100
Diagnosis/patient presentation
Patients with ulnar nerve compression in Guyon’s canal typically present with a history of paresthesias in the small and ring fingers, ulnar-sided pain, or decreased grip strength. A history of wrist trauma or vocational exposure to repetitive hand vibration is not uncommon. Patients with ulnar nerve entrapment at the wrist also frequently suffer from CTS; carpal tunnel release can often eliminate ulnar symptoms as it has been shown to decompress Guyon’s canal indirectly.101
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree