Fundamentals of Cutaneous Photobiology and Photoimmunology: Introduction
|
Knowledge of the interaction of sunlight with the skin is fundamental to understanding the pathogenesis, diagnosis, and treatment of more than 100 cutaneous disorders. Whenever ultraviolet (UV) or visible radiation is used to diagnose or treat a skin condition, important principles of photophysics involving absorption and emission of light underlie the success of the therapy. Sunscreen recommendations rely on an understanding of solar UV radiation and the ways in which the causative wavelengths can be minimized. Skin cancer is an epidemic clinical problem, whose pathophysiology necessitates comprehension of the photophysical, photochemical, and photobiologic events described in this chapter.
Almost every ancient civilization worshipped a god of the sun whose healing powers were believed to be broad reaching. Even today, sun exposure is widely felt to induce a sense of well-being. In addition, sunlight is important for the synthesis of vitamin D3 and the setting of internal clocks. On the negative side, sunlight causes deleterious acute and chronic inflammatory skin reactions, skin cancer, and photoaging, and can elicit adverse reactions to certain drugs (see Chapters 91, 92, 109, 112). Although the sun is a major source of the UV and visible radiation that interacts with human skin, UV and/or visible radiation are also emitted from common sources such as fluorescent lights, incandescent bulbs, photocopy machines, and phototherapy lamps. Tanning salons are another familiar example. Thus, UV and visible radiation are a constant part of the human environment and play a role in health, disease, and therapy. Photodermatology is the study of this interaction between human skin and UV and visible radiation. To understand the responses of skin to UV and visible radiation, it is essential to be acquainted with the principles governing the interaction of these wavebands with biomolecules in the skin.
When UV and visible light photons reach the skin surface the energy of the radiation is transformed into an observable response as shown schematically in Figure 90-1. First, the radiation must penetrate to the appropriate level in the skin where it is absorbed by molecules in the skin, termed chromophores. Photochemical reactions then convert the chromophores into new molecules, the photoproducts. These photoproduct molecules stimulate cellular signal transduction pathways leading to biochemical changes that culminate in cellular effects, such as the proliferation, secretion of cytokines, and apoptosis that are responsible for the observed acute skin responses.
Ultraviolet and Visible Radiation
UV radiation and visible light are portions of the electromagnetic (EM) spectrum, which includes a wide range of wavelengths, from high-energy X-rays to low-energy microwaves and radio waves (Table 90-1; Fig. 90-2). The UV waveband is of special interest because dozens of skin disorders are aggravated by these wavelengths and, many popular therapies, such as UVB phototherapy (see Chapter 237) and psoralen and ultraviolet A light (PUVA) photochemotherapy (see Chapter 238), use sources emitting UV radiation. Visible light encompasses those wavelengths perceived as color by the human eye and is also frequently used in therapies, such as blue-light amino levulinic acid–photodynamic therapy (PDT; see Chapter 238), and is emitted by several lasers intended to target cutaneous chromophores (see Chapter 239).
Waveband | Wavelength Range (nm) |
|---|---|
X-ray | 0.1–10 |
Vacuum ultraviolet | 10–200 |
Ultraviolet C | 200–290 |
Ultraviolet B | 290–320 |
Ultraviolet A (UVA) | 320–400 |
UVA I | 340–400 |
UVA II | 320–340 |
Visible | 400–760 |
Violet | 400 |
Blue | 470 |
Green | 530 |
Yellow | 600 |
Red | 700 |
Near infrared | 760–1000 |
Far infrared | 1000–100,000 |
Microwaves and radio waves | >106 |
For medical photobiology, the UV range (200–400 nm) is subdivided into UVA, UVB, and UVC (see Table 90-1 and Fig. 90-2). A division was made at 290 nm because wavelengths from the sun shorter than 290 nm are absorbed by ozone in the stratosphere and do not reach the earth’s surface at sea level. Wavelengths in the range of 200 to 290 nm are referred to as UVC or germicidal radiation. These wavelengths are strongly absorbed by DNA and therefore can be lethal to viable cells of the epidermis or to bacteria. UVC lamps emit at 254 nm and are used for air and water purification. Care must be taken to avoid exposure of eyes and skin to UVC radiation because of the danger of UV keratitis and mutation.
The range 290 to 320 nm is known as UVB and is often referred to as mid-UV or sunburn spectrum. It includes the biologically most active wavelengths reaching the earth’s surface. The UVB constitutes only approximately 5% of the UV and 0.5% of total radiation reaching the earth’s surface; the exact amount varies markedly with the time of day, season, cloud conditions, and other factors. Ordinary window glass blocks UVB. Most sunscreens efficiently reflect or absorb these wavelengths, and the sun protection factor (SPF) is primarily based on testing against this waveband. However, it is important to note that different wavelengths within these subdivisions can elicit greatly varying biologic responses. For example, consider the response of skin to two wavelengths in the UVB range, 297 and 313 nm. Radiation at 297 nm is nearly 100 times more erythemogenic than 313-nm radiation1 and more effectively causes DNA damage and photocarcinogenesis.2 An example relevant to phototherapy is the great efficacy of certain UVB wavelengths for treatment of full UVB spectrum.3,4 Narrowband UVB (311 nm) and excimer laser (308 nm) are used to treat psoriasis because these wavelengths are more effective than other portions of the UVB spectrum.3,4
Long-wave UV or UVA (320–400 nm) is sometimes referred to as black light because it is not visible to the human eye but causes certain substances to emit visible fluorescence. Approximately 95% of the UV radiation reaching the earth’s surface is UVA. As described for UVB radiation, the response of skin to UVA is not constant across all wavelengths from 320 to 400 nm.3 In fact, UVA has been divided into UVA I (340–400 nm) and UVA II (320–340 nm) because the latter band is more damaging to unsensitized skin than the longer wavelengths. Although most sunscreens offer their greatest protection against UVB wavelengths, those products said to be “broad-spectrum” indeed have considerable ability to protect against UVA wavelengths, although no widely used formal measure equivalent to the SPF rating yet exists. Generally, the higher the SPF, for example, 45 or greater, the more likely one is going to find some UVA protection in that preparation. Newer sunscreen agents enhance protection against damaging UVA rays (see Chapter 223). Because of its predominance in the UV radiation reaching the earth’s surface, protection against UVA is quite important for minimizing adverse cutaneous effects such as photoaging and carcinogenesis.
The visible spectrum (400–760 nm) is defined by the wavelengths that are perceived as color by the retina. Specific colors are associated with different wavelengths, as shown in Fig. 90-2. Skin responses to visible light generally require photosensitization. For example, in PDT, dyes absorbing long wavelength visible light (red) are used (see Chapter 92). Because they take advantage of the absorption spectra of endogenous chromophores, intense pulsed visible light sources such as lasers are used to treat vascular, pigmented and other lesions without application of a photosensitizing dye (see Chapter 239).
X-rays and γ rays occupy the short wavelength (high energy) end of the EM spectrum, and infrared radiation (IR) is found at longer wavelengths (lower energy) than visible radiation (see Table 90-1). X-ray and γ rays ionize molecules (remove electrons) indiscriminately and are known as ionizing radiation, the subject of radiobiology (see Chapter 240). In radiation therapy of tumors, these wavelengths kill tumor cells by ionizing water molecules and producing free radicals that damage DNA. IR has lower energy than visible light. It is subdivided into IR-A (760–1,440 nm), IR-B (1,440–3,000 nm), and IR-C (3,000 nm–1 mm). IR-A penetrates into the dermis and causes skin damage whereas IR-B and IR-C are felt as heat. Recent studies suggest that IR-A wavelengths might also be therapeutic.5
Properties of Electromagnetic Radiation
Certain principles are better illustrated by conceptualizing EM radiation as waves, whereas others are better understood by thinking of it as packets of energy called photons. These two descriptions are complementary.
When considering EM radiation as a wave, it is described as oscillating electric and magnetic fields at right angles to each other and to the direction of propagation. Consequently, it may be described either by its frequency (number of oscillations per second) or by its wavelength (distance traveled per oscillation). Frequency and wavelength have an inverse relationship, which is expressed as: ν = c/λ, where ν = frequency (number of oscillations per second), c = speed of light (3 × 108 m/s), and λ = wavelength in meters.
EM radiation also may be described as a stream of discrete packets of energy known as quanta or photons. The amount of energy in a photon (quantum) is directly proportional to the frequency of the radiation and inversely proportional to its wavelength, as expressed by Planck’s law: E = hc/λ, where E = the energy of the photon in joules (J), h = Planck’s constant (6.626 × 10−34 J/s), c = speed of light, and λ = wavelength in meters. This relationship shows that the energy of the photon increases when the wavelength is shorter and decreases when the wavelength is longer. For example, a 300-nm UVB photon has twice the energy of a 600-nm yellow photon.
Sources of Ultraviolet and Visible Radiation
The shortest wavelength of the solar spectrum reaching the earth’s surface at sea level is approximately 290 nm, although slightly shorter wavelengths are detected at high altitudes. Depending on the geographic location and the season, it has been estimated that sunlight produces between 2 and 6 mW/cm2 of UV radiation between 290 and 400 nm. Filtering of wavelengths less than 290 nm by ozone is a very important process because the shorter UVC wavelengths are highly damaging to animals and plants. Because the transmission of solar UVC and UVB through the atmosphere varies exponentially with ozone concentration, small changes in the ozone layer may result in hazardous increases in UV irradiance at the earth’s surface.6 For example, calculations of the effects of ozone depletion predicted a doubling of skin cancer incidence by 2,100 ad even if the Montreal Protocol restrictions on ozone-depleting substances were followed.7
The UV Index developed by the National Weather Service and the US Environmental Protection Agency represents an attempt to quantify the risks attendant with solar radiation at a given time and place. Among other factors, the effects of altitude, latitude, season, and clouds are considered. The scale goes up to 15, and any UV Index greater than 10 is considered a high-risk day for possible overexposure in that locale.
Skin is exposed to UV and visible radiation from a wide variety of sources in daily life and from a different set of light sources for therapy and diagnosis. Specific information should be obtained from the manufacturer before using a new light source.
Incandescent light sources include conventional electric light bulbs, flood lamps, and some quartz iodide bulbs. In these lamps, an electric current passing through a metal filament heats the filament, causing it to emit mostly visible and IR. Only the occasional patient with solar urticaria, chronic actinic dermatitis, or some porphyrias is bothered by the output of ordinary incandescent sources. Tungsten-halogen incandescent lamps, often used as flood lamps, emit UVA and visible radiation. Some quartz iodide incandescent lamps produce significant UVA and some UVB emission.
Arc sources include xenon lamps, medium- and high-pressure mercury (hot quartz) lamps, fluorescent lamps, and halide lamps. In arc lamps, electrons are driven through a gas by a potential difference between two electrodes. The gaseous molecules are ionized and subsequently release EM radiation. Radiation is emitted preferentially at specific wavelengths (emission lines) as well as in a continuum, that is, all wavelengths are emitted rather than just specific wavelengths. The wavelengths and relative power at each wavelength depend on the gas used, the arc temperature, the pressure within the lamp, and the lamp wall material.
Xenon arc lamps emit both UV and visible radiation and are now the most common sources used in solar simulators. Photoprovocation testing for polymorphic eruption is often done with such sources using filters to limit the wavelengths. Xenon arcs are also used in some phototherapy and photobiologic research applications. In a xenon lamp, xenon gas under 20–40 atmospheres (atm) of pressure produces intense visible and UV radiation. At these pressures, the xenon spectrum becomes a continuum.
The wavelengths emitted by mercury arc lamps are strongly influenced by the pressure of the gas within the envelope. Low-pressure mercury germicidal lamps emit 85% of the radiant energy at 254 nm. Because the operating temperature is low, they are also known as cold quartz lamps. With increasing pressure (1 atm), the primary 254-nm emission is absorbed by other mercury atoms within the lamp and reemitted at longer wavelengths (297, 302, 313, 334, and 365 nm, and visible wavelengths). With further pressure increases (2–100 atm), these spectral lines broaden and decrease relative to the intensity of the continuous spectral background. In medical practice, medium- and high-pressure mercury (hot quartz) lamps are generally used as sources of UVB, although their spectral power distribution is mainly in the UVA and visible range.
The commonly used UVB sunlamps (see Chapter 237) and UVA lamps for PUVA therapy (see Chapter 238) are fluorescent lamps. They are, in essence, modified low-pressure mercury arc lamps. The inner surface of the glass tube is coated with a phosphor, which absorbs the 254-nm radiation and reemits the energy at longer wavelengths. The chemical composition of the phosphor determines which wavelengths are reemitted. In general, fluorescent lamps have a relatively wide, bell-shaped emission spectra, with emission lines of mercury superimposed, and are referred to as broadband light sources.
Fluorescent sunlamps (type FS) emit mainly in the UVB range (Fig. 90-3). They are often referred to as UVB lamps, even though they emit a portion of UVA radiation, because the therapeutically significant radiation is in the UVB range (Table 90-2). A fluorescent lamp with a major emission peak at 311 nm (Philips TL01) was developed for use in phototherapy (see Fig. 90-3).8,9 This lamp is an efficient source for psoriasis phototherapy because, compared to a conventional UVB lamp, the energy emitted almost entirely overlaps with the action spectrum for clearance of psoriasis.3 Interestingly, this lamp has also been successfully used for the treatment of vitiligo, atopic dermatitis, and polymorphic light eruption, all of whose action spectra are unknown and may possibly differ from that for psoriasis.
Figure 90-3
Emission spectra of some of the fluorescent lamps used in phototherapy. A. Broadband ultraviolet B (UVB) fluorescent lamps and UVA (black light) fluorescent lamps. B. Narrowband (Phillips TL01) fluorescent lamp with a maximum at 311 nm and a fluorescent lamp with a Wood’s glass filter. C. UVA I halide lamps (Sellamed 24,000 bed system). Emission spectra were measured with a Luzchem model SPR-4001 spectroradiometer.
% UVB | % UVA | |
|---|---|---|
Broadband UVB (Westinghouse Sunlamp) | 60 | 40 |
Narrowband UVBa (Phillips Fluorescent UVB, TL01, 311 nm) | 80 | 20 |
Broadband UVA (Houva Lite; Black Light by Sylvania, Westinghouse, and General Electric; Sylvania Puva Life Line; Dermalight Metal) | 2 | 98 |
UVA Ib (Dermalight UltrA1, Dr. K. Hönle Medizintechnik GmbH) | 0 | 100 |
Wood’s lightc (RA Fisher; Spectronics) | 0 | 100 |
Halide lampsd (Dermalight Systems, Dr. K. Hönle Medizintechnik GmbH) | 0 | 100 |
High-intensity, UVA-emitting fluorescent lamps are most often used in PUVA therapy of psoriasis, vitiligo, and other skin diseases. The most efficient UVA source for PUVA therapy would maximize the emission of 320- to 360-nm radiation for photoactivation of psoralen molecules, while minimizing the UVB emission. Table 90-2 shows the percentage of total emission that is in the 320–360-nm range from several UVA-emitting light sources that are used in phototherapy units.
Wood’s lamps are small, low-pressure, UVA-emitting fluorescent lamps with a UVA-transmitting, visible-absorbing glass envelope. They are useful in clinical practice because, after UVA absorption, the fluorescent emission from normal and abnormal components of skin, hair, teeth, and urine may be diagnostic (e.g., in porphyria, vitiligo, and fungal infection).
Halide lamps emit a high-intensity continuum in the UVB and especially the UVA range. With appropriate filters, this lamp is used increasingly as a UVB, as well as a UVA, source for phototherapy (in particular, UVA I therapy) and photochemotherapy. The metal halide lamp usually consists of a high-pressuremercury lamp with metal halides as additives. The continuous range of wavelengths emitted from halide lamps differentiates them from medium-pressure mercury arc lamps that emit in narrow wavelength ranges. Approximately 20% of the emission spectrum can be UVA radiation.
(See Chapter 239.)
Lasers produce intense beams of monochromatic (single wavelength) radiation. The laser operates by exciting molecules to a metastable excited state from which photon emission is stimulated by a subsequent photon incident on the excited molecule. The emitted photon and the stimulating photon are then each capable of stimulating emission of yet other excited molecules, eventually producing an avalanche of photons of the same wavelength, phase, and direction of propagation. Different lasers emit UV, visible, or infrared wavelengths and may operate as either continuous or pulsed sources.
Ultraviolet and Visible Radiation Dosimetry
To treat patients with the appropriate amount and wavelengths of UV or visible radiation, it is important to become acquainted with how the radiation is quantified and related to the exposure time. The basic unit of EM energy is the joule (J). Power is the rate of energy flow, joules per second (J/s), usually expressed as watts (W). The rate at which the radiant energy is delivered to a surface, such as skin, is expressed as the power delivered per unit area of surface (power/area; W/cm2). This quantity is called irradiance. The total radiant energy delivered per unit area of skin surface over a period of time is called the exposure dose or fluence and is the product of irradiance and time:
For most responses to UV and visible radiation, it is the fluence at particular wavelengths that determines the magnitude of the response. The irradiance generally does not affect the response using conventional light sources.
The irradiance delivered by a source as a function of wavelength is called the spectral irradiance and is expressed as units of irradiance per nanometer [(W/cm2)/nm]. A spectroradiometer is used to measure the spectral irradiance of a light source. When measuring a light source’s irradiance over a given spectral region, a detector weighted to the wavelengths of interest is most useful. For example, a broadband radiometric measurement of wavelengths less than 315 nm provides a rough indication of the erythemally effective wavelengths emitted by a source of UV radiation.
There is, however, no substitute for knowing the full spectral irradiance delivered by a source, as determined by a spectroradiometer. For example, in phototesting, only the wavelengths of interest should be used. To assess endogenous UVA sensitivity, the UVB portion of a source’s emission, if present, must be filtered out so that the more erythemogenic UVB wavelengths do not lead to a falsely lower determination of erythema threshold in the UVA range.
Optical Properties of Skin
When UV and visible radiation strike the skin, part is remitted (reflected and scattered), part is absorbed by chromophores in various layers, and part is transmitted inward to successive layers of cells until the energy of the incident beam has been dissipated (Figs. 90-4 and 90-5). The two major processes limiting the penetration of UV and visible radiation into skin, absorption and scattering, vary with wavelength.10
UV wavelengths less than 320 nm are readily absorbed by proteins, DNA, and other components of epidermal cells. Along with scattering, this absorption accounts for the low penetration of these wavelengths into skin (see Fig. 90-4). For example, approximately 10% of 300-nm radiation and 50% of 350-nm radiation reaches the dermal–epidermal junction in fair skin. Between 5% and 10% of incident light is reflected by the outer surface of the stratum corneum. This surface or so-called specular reflectance is relatively constant for all visible wavelengths and accounts for the surface appearance of skin, which is especially glossy if the surface is smooth, wet, or oily. In contrast, if the surface is irregular, the light is scattered and the skin appears dull or “rough.” Moisturizers applied to the skin reduce this rough appearance by smoothing out the many air–surface interface irregularities and making the skin look shinier.
Scattering includes any process that deflects the path of optical radiation. For example, skin with scales, as in psoriasis, scatters more light than does normal skin. During phototherapy, application of emollients to the psoriatic plaques helps reduce the scattering of UV radiation and allows more of the effective wavelengths to penetrate into the viable tissue.
Melanin, which absorbs relatively uniformly over the visible wavelengths and is normally present only in the epidermis, acts largely as a neutral density filter to diminish remittance from the dermis. The greater overall melanin content in darker skin absorbs more visible light and therefore causes the skin to appear darker as there is less light remitted back to the observer. Blood (hemoglobin) within the dermis absorbs the shorter (blue) visible wavelengths, diminishing these spectral regions, and giving a reddish hue to our perception of the total remittance. Abnormal location and quantity of these or other pigments account for the appearance of the skin in pathologic conditions (e.g., melasma with extra pigment in the epidermis and/or dermis, vitiligo with an absence of epidermal melanin).
Absorption of Ultraviolet and Visible Radiation by Molecules in Skin
When a photon is taken up by a chromophore, it is said that absorption has occurred. The specific wavelengths absorbed by each molecule (called an absorption spectrum) are characteristic of the structure of the molecule (i.e., the arrangement of the nuclei and electrons). Only radiation that is absorbed by the chromophores can initiate biologic responses.
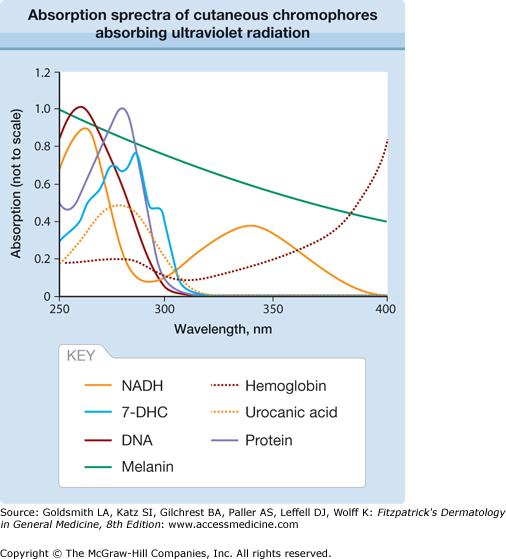
The absorption spectrum of a compound is a plot of the probability of absorption of photons of a specific wavelength on the Y-axis against wavelength on the X-axis (Fig. 90-6). The wavelengths that have the highest probability of absorption are called absorption maxima, λmax, (e.g., DNA, λmax = 260 nm; porphyrins, λmax = 400–410 nm). Many of the biomolecules that absorb in the UVB spectrum actually have absorption maxima at shorter wavelengths in the UVC range (see Fig. 90-6). These include the purine and pyrimidine bases in DNA and RNA (λmax ∼260 nm) and 7-dehydrocholesterol (λmax = 285 nm). Many endogenous cutaneous chromophores absorb UVA or a combination of UVA and visible radiation, including hemoglobin (λmax = 410 nm) and bilirubin (λmax = 450 nm). Melanin absorbs throughout the UV, visible, and near IR spectra without a distinct absorption maximum wavelength. The absorption spectra of few chromophores extend into the IR-A waveband, an exception being cytochrome c oxidase, which is last enzyme in the mitochondrial respiratory electron transport chain.
Figure 90-6
Absorption spectra of cutaneous chromophores absorbing ultraviolet radiation. Note that the relative amounts of ultraviolet radiation absorbed by these chromophores in skin depend on the heights of the absorption peaks as shown in this figure, the amount of each chromophore in skin, and the penetration of each wavelength into skin. 7-DHC = 7-dihydrocholesterol; NADH = reduced nicotinamide adenine dinucleotide.
Photochemical Reactions Leading to Skin Responses
After absorbing the energy of a photon, the chromophore is in an “excited state,” which exists for only a very short time before reacting with nearby molecules. The products of these reactions initiate signal transduction processes that lead to the observed responses in skin.
Normally, molecules are in the so-called ground state and have a certain distribution of electrons in space around the nuclei of their atoms. For each molecule, a series of electronic states with higher energies and different distributions of electrons also exist; these are called excited states. When a molecule in the ground state absorbs the energy of a UV or visible photon, the molecule is promoted to an excited electronic state (Fig. 90-7). According to quantum mechanics, only certain energy gaps are allowed to exist between electronic states. Consequently, a molecule can absorb photons only with certain energies; this results in a unique absorption spectrum for each molecule.
A molecule exists in this first excited state formed for a very brief period. It is called a singlet excited state and exists for a few nanoseconds. The molecule may return to the ground state by emitting light (fluorescence) or releasing the energy as heat by a process called internal conversion (see Fig. 90-7). Alternatively, the singlet excited state may undergo a chemical reaction to form a photoproduct, or it may convert into another excited state with lower energy, the triplet excited state, by a process called intersystem crossing (see Fig. 90-7). Singlet and triplet excited states differ in the spins of a pair of electrons in an orbital. The ground state is almost always a singlet state. The triplet excited state may exist for a longer time (i.e., a few microseconds). It may emit light (phosphorescence), undergo a chemical reaction, or return to the singlet ground state by intersystem crossing (see Fig. 90-7).
These excited state processes are responsible for the effectiveness of light for diagnosis and therapy. For example, fluorescence occurs every time a Wood’s light is used. UVA emitted from this lamp causes autofluorescence of dermal collagen fibers. To the examining physician, this fluorescence is viewed through the overlying epidermis. Thus, any epidermal lesions such as lentigines tend to have their borders accentuated by contrast because the fluorescence is observed most brightly around the lesion. The heat generated by internal conversion is responsible for the effects of pulsed lasers (see Chapter 239).
During a photochemical reaction, the excited state molecule is transformed into a new, stable molecule called the photoproduct. Photoproducts in DNA are important for UVB-induced responses in skin. The photoproduct molecule may be produced entirely by rearrangement of the bonds in the chromophore, for example, the formation of previtamin D3 from 7-dehydrocholesterol or in DNA of a four-membered ring structure called a cyclobutyl pyrimidine dimer (CPD) from adjacent thymines or cytosines. These DNA photoproducts lead to mutations and development of nonmelanoma skin cancers (see Chapter 112). Alternatively, the chromophore may form covalent bonds with another molecule in the cell, for example, the formation of covalent adducts between psoralen and DNA. The phototherapy of diseases such as psoriasis makes use of this photochemical reaction (see Chapter 238). In addition, many skin responses to UV and visible light are initiated when the excited state chromophore transfers its energy to oxygen to form singlet oxygen or transfers an electron to form superoxide anion. These forms of oxygen react with cellular molecules, which often initiates intracellular signal transduction leading to the inflammation seen in sunburn and drug phototoxicity.
Photochemical reactions vary in efficiency. Not every chromophore molecule that absorbs a photon undergoes a photochemical reaction because the excited states may fluoresce or follow other pathways (see Fig. 90-7). The term quantum yield indicates the likelihood that one of these processes occurs. For example, the quantum yield for forming a certain photoproduct is:
When certain drugs (e.g., tetracyclines, fluoroquinolones, psoralens) and dyes absorb UV and/or visible radiation, delayed erythema or inflammation is observed (see Chapter 92). This phenomenon is called photosensitization, and the dyes and drugs are called photosensitizers. Photosensitization requires the presence of oxygen in most cases and the initial photoproducts are reactive oxygen species (ROS). ROS are small molecules and free radicals including singlet oxygen, hydrogen peroxide, superoxide anion and hydroxyl radical. These ROS oxidize unsaturated lipids in membranes, certain amino acids in proteins (histidine, methionine, tryptophan, cysteine), and guanine in nucleic acids. The oxidized products initiate signal transduction processes leading to inflammatory mediators, for example, prostaglandin E2 (PGE2) and tumor necrosis factor (TNF)-α, that produce the inflammation observed as erythema.
A photosensitizer molecule in an excited triplet state can transfer its energy to an oxygen molecule, thereby generating the ROS called singlet oxygen (1O2). Singlet oxygen is relatively long-lived for a singlet state (<4 microseconds) and reacts with other cellular molecules to generate additional ROS. The skin photosensitization produced by PDT drugs and by protoporphyrin IX, the porphyrin that accumulates to abnormally high levels in red blood cells of patients with erythropoietic protoporphyria (EPP), involves initial formation of singlet oxygen. Oxidation of membrane lipids is believed to initiate the signal transduction processes, leading the wheal and flare response observed in EPP photosensitivity. Singlet oxygen is also involved in the phototoxicity mechanisms for some phototoxic drugs. However, the observed skin phototoxicity responses often differ from that seen in EPP photosensitivity, possibly because of the presence of the photosensitizing agents in different tissue locations (e.g., epidermis, dermis, or vasculature). The photosensitizers may also be in different locations in the cells (i.e., nucleus, mitochondria, or cell membranes), which would influence the photoproducts formed and the subsequent cutaneous effect observed. Endogenous UVA-absorbing chromophores generate singlet oxygen in keratinocytes and fibroblasts and in skin.11
Endogenous chromophores and some exogenous photosensitizers generate other ROS.12 For example, hydrogen peroxide and superoxide anion are produced after photosensitized and direct photon damage to mitochondria. The UVB or UVA radiation also activates ROS-producing enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in keratinocytes and fibroblasts.13,14 The UVA-induced responses in normal skin and in photosensitivity conditions are generally dependent on production of ROS. Recent studies suggest that ROS are important in the development of many UVB-initiated responses. Antioxidants act by quenching (i.e., chemically reacting with and removing) ROS and other free radicals before they can damage cellular molecules.
Acute Responses to UV Radiation
Sunburn and tanning are the most obvious responses elicited by exposure of skin to a single dose of UV radiation. Many other responses are less visually apparent, but have important physiologic effects including formation of vitamin D3, altered immune responses, production of antimicrobial peptides and disruption of the epidermal barrier function. In addition, the cumulative effect of repetitive exposures gives rise to chronic skin changes including skin cancer development and photoaging. All of these responses result from a complex burst of UV-induced activity in the epidermis and dermis involving cytokines, neuropeptides, prostaglandins, ROS, and altered expression of at least 600 proteins.11,15–17
This section centers on three different parts: (1) UV-induced inflammation (sunburn), (2) vitamin D synthesis, and (3) altered immune responses. Two other acute responses to UV, (1) tanning (melanogenesis) and (2) abnormal acute responses to UV, are described in Chapters 72 and 91, respectively.
Erythema is the most visually apparent indicator of UV-induced skin inflammation. Increased blood flow in the superficial and deep vascular plexus is responsible for the appearance of erythema and also may increase skin temperature. Two other classical signs of inflammation, swelling caused by increased vasopermeabilty and pain caused by released mediators on nerve endings, may also occur. Both UVB and UVA radiations elicit inflammation although the initiating photochemistry, cell signaling processes, and biochemical pathway processes involved are not identical. Erythema has been used as the endpoint for measuring the relative effects of different wavelengths in the UVB and UVA, usually expressed as an action spectrum.
An action spectrum indicates which wavelengths most effectively produce a skin response. It is used to understand the basic science of a photobiologic as well as a therapeutic response because phototherapy is most efficient when the emission of the lamp matches the action spectrum for the beneficial response. An action spectrum is most accurately plotted as the reciprocal of the number of incident photons required to produce a given effect (Y-axis) against wavelength (X-axis). Conventionally in dermatology, the reciprocal of the minimum fluence (exposure dose) to produce the response is plotted versus wavelength. In an ideal case, the action spectrum corresponds to the absorption spectrum for the chromophore and can be used to identify the chromophore for a given photobiologic reaction. For example, an action spectrum for wheal formation in a patient with EPP showed a maximum at 400 nm, close to the maximum of protoporphyrin IX, which supports biochemical evidence for this porphyrin as the chromophore.18 Another example involves the action spectrum for the clearance of psoriasis, which was found to peak around 313 nm.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree