Fig. 1.1
Relationship between different time periods surrounding the menopause. (Reproduced, with permission of the publisher, from World Health Organization Scientific Group. Research on the menopause in the 1990s [5])
1.2.1 Menopause
Menopause is the most identifiable event of the perimenopausal period and should be characterized as an event rather than a period of time. The most widely used definition for natural menopause is as defined by the World Health Organization as at least 12 consecutive months of amenorrhea not because of surgery or other obvious causes [5]. When referring to menopausal age or onset of menopause in this chapter, we mean natural menopause as defined above. This cessation of menses resulting from the loss of ovarian function is a natural event, a part of the normal process of aging, and is physiologically correlated with the decline in estrogen production resulting from the loss of ovarian follicular function and therefore represents the end of a woman’s reproductive life.
1.2.2 Perimenopause
The perimenopausal period includes the time before, during, and after menopause, when the endocrinological, biological, and clinical features of approaching menopause commence. The years immediately preceeding and the decades afterward, however, are of far greater clinical significance. The length of this period varies, but it is usually considered to last approximately 7 years, beginning with the decline in ovarian function in a woman’s 40s and continuing until she has not had a menstrual period for 1 year [6]. Perimenopause usually begins in the mid- to late 40s; it is often uneventful but may be abrupt and symptomatic. The term “climacteric” should be abandoned to avoid confusion. Symptoms that begin with the menopausal transition usually continue into the postmenopausal period.
1.2.3 Menopausal Transition
The period of hormonal transition that precedes menopause is sometimes known as the menopausal transition period and is characterized by a varying degree of somatic changes that reflect alterations in the normal functioning of the ovary. Early recognition of the symptoms and the use of appropriate screening tests can minimize the impact of this potentially disruptive period [6]. In many cases, however, it is difficult to differentiate stress-related symptoms from those associated with decreasing levels of estrogen. For this reason, both stress and relative estrogen deficiency should be considered when managing problems associated with the menopausal transition.
In some women, menstrual irregularity is the most significant symptom of the menopausal transition [7]. Because abnormal bleeding is one of the most common symptoms of uterine problems, menstrual irregularity during the perimenopause should be evaluated carefully. Often uterine bleeding associated with this transition period is secondary to normal physiologic estrogen fluctuations rather than underlying pathology and may be treated medically [8].
1.2.4 Postmenopausal Period
The postmenopausal period is one of relative ovarian quiescence following menopause [4, 6]. Given the current lifespan of women in the United States, this period can comprise more than one-third of the average woman’s life. During this prolonged period, women are susceptible to health problems associated with estrogen deficiency that tend to be chronic rather than acute. First of all osteoporosis is not clinically apparent until decades after menopause, when unfortunately it becomes harder to treat. Additionally, the impact of estrogen deficiency on cardiovascular disease is often confused with age-related changes, while, because of the peripheral conversion of both ovarian and adrenal androgens to estrogen, the loss of ovarian function does not result in an acute estrogen deficiency in all women.
1.2.5 Time of Natural Menopause
Natural menopause occurs at a median age of 51.4 years and is more or less normally distributed with a range roughly between 42 and 58 years [7, 9, 10]. However, there is no way to predict when an individual woman will have menopause or begin having symptoms suggestive of menopause. The average age of menopause has remained invariable during the last decades.
Environmental factors explain only a small part of the age variance at which menopause commences [11]. The variation in natural menopause is a trait predominantly determined by interaction of multiple genes, whose identity and causative genetic variation remains to be determined. Based on the fact that there is a strong association between age at menopause between mothers and daughters, it is suggested that there might be a largely genetically determined trait [12]. Furthermore, the onset of menopause does not appear to be related significantly to race, parity, height, weight, socioeconomic status, nutritional status, or age at menarche [13]. On the other hand the interaction among environmental factors such as smoking (known to accelerate the age of menopause by 1.5–2 years), body mass index (BMI), alcohol use, and socioeconomic status and genetic risk may be important [14]. As a result, it has been noticed that menopause occurs earlier in nulliparous women, in tobacco smokers, and in some women who have had hysterectomies [11, 15].
1.2.6 Induced Menopause
There are some medical and surgical conditions that can influence the timing of menopause. The term induced menopause is defined as the cessation of menstruation which follows either surgical removal of both ovaries or iatrogenic ablation of ovarian function by chemotherapy or radiation.
1.2.6.1 Surgical Menopause
It is called the surgical removal of the ovaries (oophorectomy) throughout reproductive period and results in an immediate cessation of estrogen production. In more than 40 % of women who have hysterectomies, both ovaries are removed, and this is usually performed at a significantly younger age than the age of natural menopause. In this case, there is no perimenopause, and after surgery, hot flashes and other acute symptoms associated with the perimenopausal period often become especially intense [15]. In addition, long-term surgical menopause has been associated with significantly higher risk for osteoporosis than has natural menopause [16]. On the other hand, recent data suggest that surgical menopause is not a key determinant of cardiovascular disease (CVD) risk factor status either before or after elective surgery in midlife [17]. These results should provide reassurance to women and their clinicians that hysterectomy in midlife is unlikely to accelerate the CVD risk of women, in contrast to older reports that women with a hysterectomy had a worse risk profile and higher prevalence and incidence of CVD [18]. If a hysterectomy is not accompanied by the removal of both ovaries in a woman who has not yet reached menopause, the remaining ovary or ovaries are still capable of normal hormone production. In this case, a woman cannot menstruate but hormonal production from the ovaries can continue up until the normal time when menopause would naturally occur. At that time women could report the other symptoms of menopause such as mood swings and hot flashes, which are not therefore associated with the cessation of menstruation.
1.2.6.2 Cancer Chemotherapy and Radiation Therapy
Chemotherapy and/or radiation therapy in a woman of reproductive age can result in menopause. The effect of such a treatment on ovarian function is directly depended on the type and location of the cancer as well as the toxicity of the medications used [19]. In this case, the symptoms of menopause may begin during the cancer treatment or may develop in the months following the treatment, independently of the woman’s age.
1.2.7 Premature Menopause
Premature menopause or premature ovarian failure (POF) is defined as the spontaneous occurrence of menopause before the age of 40, occurring in 0.1 % of women under 30 years of age and 1 % of women by age 40 [20, 21]. This definition is rather arbitrary, because it is based on age only. POF is a collective term for which proposed causes include autoimmune disease, syndromes such as fragile X, or inherited (genetic) factors [22]. Genetic factors are thought to have a strong association with POF. Among patients with idiopathic POF, a higher incidence of family history of early menopause and infertility has been noted so that a familial transmission is observed in 30–40 % [23]. Although inheritance appears to be either X-linked or autosomal dominant sex limited, paternal transmission cannot be excluded. Furthermore, women with POF have a genetic pattern similar to whose with idiopathic early menopause (between the ages of 40 and 45), suggesting the existence of common underlying causal factors in both entities. Women with premature menopause are at risk of premature death, neurological diseases, psychosexual dysfunction, mood disorders, osteoporosis, ischemic heart disease, and infertility. Public enlightenment and education is important tool to save those at risk [24].
1.3 Physiology of the Normal Menopause
The ovary is unique in that the age associated with decline in function (to complete failure) appears to have remained relatively constant despite the increase in longevity experienced by women over the last century [25]. The primary determinant of reproductive age in women is the number of ovarian nongrowing (primordial, intermediate and primary) follicles (NGFs). The leading theory regarding the onset of menopause relates to a critical threshold in oocyte number and particularly the number of ovarian follicles present in the ovary. Therefore, the number of ovarian granulosa cells available for hormone secretion appears to be the most critical determinant of age at menopause, steroid hormone secretion, and gonadotrophin levels [26].
Human follicles begin their development during the fourth gestational month. Approximately 1,000–2,000 germ cells migrate to the gonadal ridge and multiply, reaching a total of five to seven million around the fifth month of intrauterine life [27, 28]. In female fetus, between the 12th and 18th week, the germ cells will enter meiosis and differentiate so that all germ stem cells have differentiated prior to birth. At this point, replication stops and follicle loss begins so that the population of NGFs is estimated to be approximately 500,000–1 million at birth, which represents the initial NGF endowment in women. At menarche 500,000–600,000 follicles exist, while in the adult woman through a combination of recruitment toward dominant follicle development and ovulation or atresia, the stock of NGFs is depleted [29, 30]. The pioneering work of these investigators led to the understanding that ovarian follicle number decreases with increasing age and that ultimately few, if any, follicles remain following menopause [31, 32].
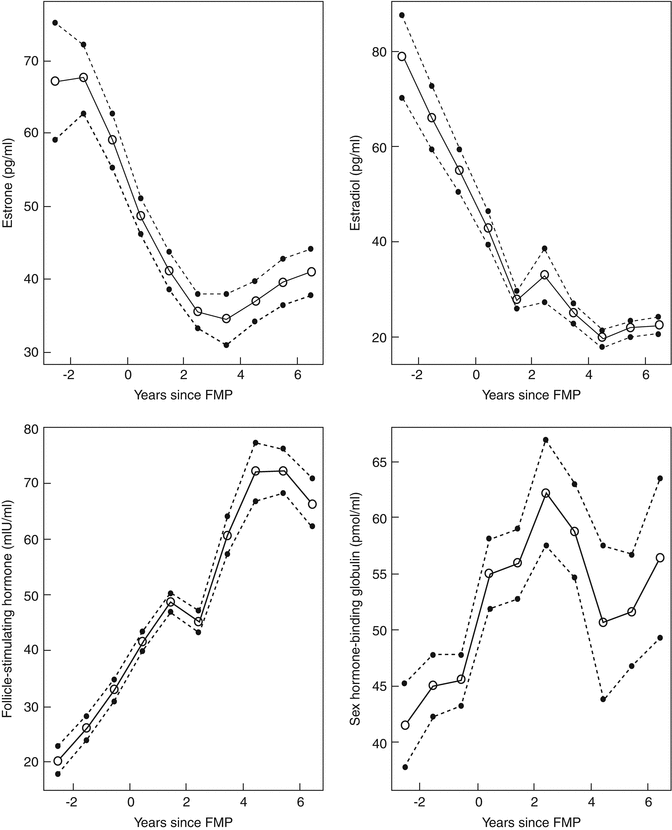
Using the combined data from these studies, it has been suggested that the decline in ovarian follicles associated with aging was best described by a biphasic-exponential model, which was better fitted to the data than either a linear or single exponential model, as shown in Fig. 1.2 [33]. In this model, the total follicular endowment at birth is estimated to be 952,000, with an initial rate of decay of −0.097. At the age of 38 years and a follicle count of 25,000, a sudden increase in decay occurs to over twofold the initial rate (−0.237). At this point, the rate of follicular atresia accelerates. In the absence of this acceleration, the model suggests menopause would be delayed until age 71. The unexpectedly faster rate of ovarian aging afterwards lowers the follicle population to 1,000 at approximately 51 years and is adopted as the menopausal threshold as it corresponds to the median age of menopause. The cause of this accelerated depletion is not well defined. It is also clear that if the factor influencing the rate of decline is follicle number and not age, other factors which might account for a diminished follicle number (genetic risk and possible toxic exposure) would lead to an earlier rate of accelerated decline and an earlier age of menopause.
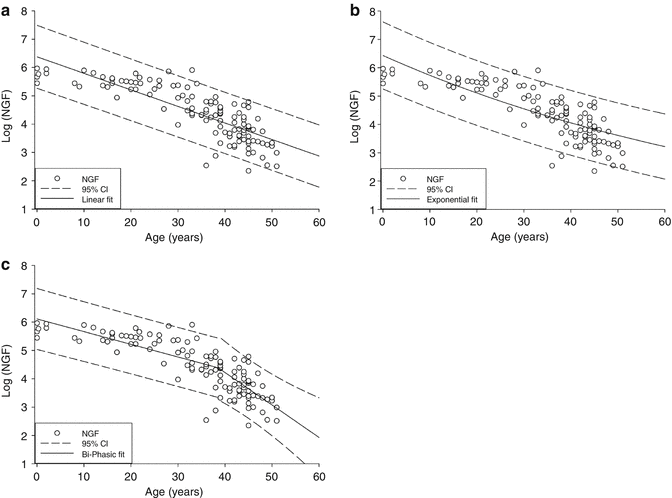

Fig. 1.2
Models of ovarian NGF decay. The log of the ovarian NGF number is plotted versus age (years). (a) Linear model, (b) exponential model, and (c) biphasic-exponential model. Solid lines indicate the fitted model with dashed lines representing the 95 % confidence interval (n = 122) (Reprinted from Hansen et al. [26], by permission of Oxford University Press)
Realizing the biological implausibility of a sudden acceleration in follicular depletion, a newer power model has been proposed [26]. This power model suggests that the decay of NGFs is constantly accelerating rather than suddenly increasing at 38 years as shown in Fig. 1.3. It suggests that age accounts for approximately 84 % of the variation in NGF count at different ages and agrees well with the known distribution of menopausal age but also is more biologically plausible than previous models.
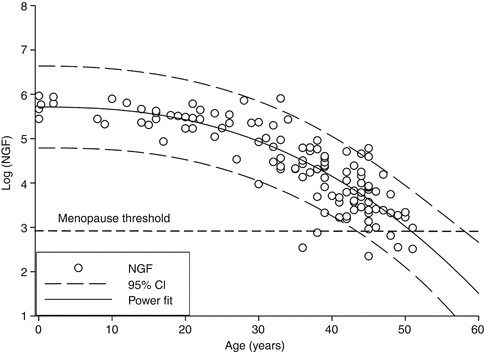

Fig. 1.3
Power model of ovarian NGF decay. The log of the ovarian NGF number is plotted versus age (years). The solid line indicates the fitted model with dashed lines representing the 95 % CI (Reprinted from Hansen et al. [26], by permission of Oxford University Press)
The biology underlying the transition to menopause includes not only the profound decline in follicle numbers of the ovary but also a significant increase in random genetic damage within the ovaries. This is supported by the fact that in women over 40 years old, oocytes harvested for in vitro fertilization are karyotypically abnormal approximately 40 % of the time [34]. At the same time an increase in aneuploidy in the offspring of older mothers confirms the potential role of a genetic ovarian defect, while new information will have to accumulate before the factors governing human oocyte atresia are elucidated more clearly.
1.4 Endocrinology of the Normal Menopause
Menopause and the years preceding are characterized by cessation of ovarian function, concomitant hormonal changes, and monthly menstruation and are associated with the end of reproductive capability. The biological basis for these events is well established, being dependent on changes in ovarian structure and function. During the menopause, there is a reciprocal relationship between ovarian hormone levels, which decline, and pituitary gonadotrophins, which increase (Fig. 1.4) [35].
The ovarian hormones are divided into two classes: the steroids, primarily estradiol and progesterone and secondary androgens, and the peptides, primarily inhibins and activins. Estradiol and the peptide hormones are secretory products of the ovarian granulosa cells, the major cell type of ovarian follicle, whereas progesterone is a product of the corpus luteum. The primary biological properties of the peptide hormones are implied by their names; inhibin suppresses synthesis and secretion of pituitary follicle-stimulating hormone (FSH), whereas activin stimulates FSH secretion. In addition to FSH, the other relevant pituitary hormone is luteinizing hormone (LH), whose secretion is controlled primarily by the steroid hormones, whereas FSH is regulated by both the steroids and the peptide hormones [36].
FSH is an established indirect marker of follicular activity. In studies of groups of women, its concentration, particularly in the early follicular phase of the menstrual cycle, begins to increase some years before there are any clinical indications of approaching menopause [37]. The rise in FSH is the result of declining levels of inhibin B (INH-B), a dimeric protein that reflects the fall in ovarian follicle numbers, with or without any change in the ability of the lining granulosa cells to secrete INH-B. Early on, there is also a decline in luteal phase progesterone levels. As ovarian aging progresses, estradiol levels may be quite variable, with chaotic patterns and, occasionally, very high and very low levels. This dramatic variability may lead to an increase in symptomatology during the perimenopause. As peripheral gonadotrophin levels rise, LH pulsatile patterns become abnormal. There is an increase in pulse frequency with a decrease in opioid inhibition. As a result, during the transition, hormone levels frequently vary markedly – hence, measures of FSH and estradiol are unreliable guides to menopausal status.
1.4.1 Estrogens
The main circulating estrogen during the reproductive years is 17β-estradiol. Estradiol levels range from 50 to 300 pg/mL and are controlled by the developing follicle and resultant corpus luteum. It is estimated that 95 % of circulating estradiol is derived from the ovary, based on the fact that surgical removal of the ovaries reduces peripheral estradiol levels from 120 to 18 pg/mL. Additionally small amounts of estradiol are produced by the adrenal gland as well as by the peripheral conversion of testosterone and estrone [25].
Even though the amount of estrogen secreted by the postmenopausal ovary is insignificant, postmenopausal women continue to have measurable amounts of both estrone and estradiol [38]. Estrone and estradiol production rates in postmenopausal women are 40 and 6 μg/day, compared to 80 and 500 μg/day, respectively, during the reproductive years. After menopause the main circulating estrogen is estrone, which has a biologic activity approximately less than 30 % of estradiol activity and is mostly derived from peripheral conversion of androstenedione. Extraglandular aromatase is found in liver, fat, and some hypothalamic nuclei, and its activity increases with aging as well as with a higher fat content. Estradiol in the postmenopausal women is derived mainly from conversion of estrone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree