Definition
Generalizeda
Localizedb
Provoked
Present only with contact, sexual or nonsexual
✓
✓
Unprovoked
Present without contact
✓
✓
Mixed
Provoked & unprovoked
✓
✓
While the earliest accounts describing symptoms of vulvodynia were found even in the first century [5], the term “vulvodynia” was suggested by Tovell and Young in 1978 [6]. Later in 1987, Dr. Edward Friedrich coined the term “vulvar vestibulitis” along with the diagnostic criteria known as Friedrich’s criteria. These criteria include (1) severe pain in the vulvar vestibule upon touch or attempted vaginal entry, (2) tenderness to pressure localized within the vulvar vestibule, and (3) vulvar erythema of various degrees [7]. From 1987 to 2003, different definitions and classifications of vulvodynia were discussed, including vulvar dermatoses, vulvitis (cyclic candidiasis), vulvar papillomatosis, essential vulvodynia, dysesthetic vulvodynia, and vulvar vestibulitis syndrome (VVS) [8]. The confusion was the result of disagreement as to whether vulvodynia was a symptom (secondary to infection, inflammation, neoplasm, etc.) or a primary disorder. The 17th Congress of the ISSVD established the current definition and classification system of vulvodynia 2003, published in 2004 [1]. This classification separated chronic vulvar pain into two categories: (1) vulvar pain due to identifiable medical causes and (2) vulvar pain in the absence of any known medical cause.
21.3 Epidemiology
Estimates of the prevalence of vulvodynia range from 4 to 16 % over a woman’s lifetime [9, 10]. In response to a self-administered questionnaire sent to ethnically diverse women in Boston, 16 % of respondents reported a history of burning vulvar pain for at least 3 months, and 7 % had the pain at the time of the survey [9]. A population-based survey in southeastern Michigan reported that 8.5 % of women over the age of 18 years were currently suffering with vulvodynia [10], and a New Jersey study found a 9.9 % lifetime prevalence with 3.8 % of respondents reporting current pain [11].
The occurrence of vulvodynia may be underestimated because of frequent misdiagnosis. Both physicians and affected women are frequently not aware of this condition; they either persistently or even desperately look for an organic cause to the symptoms or dismiss it as a psychological “all in the head” issue. In the Boston survey, 40 % of symptomatic women chose to not seek treatment, and those who did often saw three or four physicians without a correct diagnosis [9].
Vulvodynia affects both reproductive and nonreproductive-aged women; however, statistics of vulvodynia in postmenopausal women are lacking. In a large population-based sample (N = 12,435), Harlow showed that 64 % of patients with vulvodynia were aged between 18 and 35, 22 % between 45 and 54, and 14 % between 55 and 64 [12]. Most of the patients with provoked vestibulodynia (the most frequent pattern of vulvodynia) are premenopausal, the mean age being 27.8 years old in a recent study [13]. Unprovoked vulvodynia is more likely to occur in postmenopausal women; in a series of 159 patients with unprovoked, most frequently generalized, vulvodynia, the mean age was 56 (Moyal-Barracco M, unpublished data).
Menopausal women with chronic vulvar pain are frequently diagnosed with vulvovaginal atrophy (VVA). A 2013 Internet-based survey of postmenopausal women designed to assess the effects of VVA found 44 % of respondents experienced dyspareunia and 37 % experienced vulvar irritation (N = 3046). Although the most common symptom was vaginal dryness (55 %), the study suggests a definite need for evaluation and treatment of postmenopausal women with vulvovaginal symptoms [14]. Genital atrophy due to loss of estrogen is not the only cause of vulvar discomfort in menopausal women, and likely evaluation of women with these complaints will also uncover other etiologies such as vulvodynia. Though the onset of vulvodynia is usually during reproductive years, it may persist or even start in the postmenopausal years. Postmenopausal women complaining of persisting discomfort in the absence of visible findings, despite treatment with hormonal replacement therapy either local or systemic or both, should be considered to possibly suffer from vulvodynia [15].
21.4 Vulvodynia and Menopause: Physiological Considerations
The age-related morphological and physiological changes of the vulva and vagina over a lifetime are well established, as is the hormonal mediation of these events [16]. At birth, the vulva and vagina exhibit the effects of residual maternal estrogens which dissipate by the fourth postnatal week [17, 18]. During puberty, adrenal and gonadal steroid hormones induce maturation of these tissues [19], which continue to undergo changes during the reproductive years, linked to the menstrual cycle and pregnancy [20]. At menopause, there is a dramatic loss of estrogen, which leads to vulvar and vaginal atrophy. Pubic hair becomes sparse, the labia majora loses subcutaneous fat, and the skin of the vulva thins. The vaginal mucosa loses glycogen, with a subsequent rise in the vaginal pH and decrease of vaginal secretions. Decreased vaginal blood flow and pelvic floor muscle tone also occurs [21–23]. Symptoms are variable among women, but may include dyspareunia, irritation, burning, or itching [24]. As such, the symptoms of VVA may mimic those of vulvodynia.
Perimenopause, the transition period to menopause, usually begins at the median age of 45 years and lasts for about 4 years. Perimenopause is characterized by menstrual cycle irregularity, with an increase in the number of anovulatory cycles. The symptoms vary and include cramps, bloating, and breast tenderness as well as hot flashes, migraine, and vaginal dryness [25]. Menopause is established 1 year after the last menstrual period [26].
Estrogens also affect many levels of the pain pathway, including the tissue inflammatory response, sensory neurons and dorsal root ganglia, spinal cord, supraspinal centers such as opioidergic/serotonergic pain modulation systems, limbic circuits for affective states, and stress responses [27]. Estrogen receptors present in the central and peripheral nervous systems are known to influence all aspects of neural activity from membrane permeability to gene regulation. The transition into menopause, with the accompanying change in systemic estrogen concentration, may therefore affect chronic pain [22].
21.5 Diagnosis
The evaluation of postmenopausal women with vulvar pain includes a detailed history and a targeted physical exam. Vulvodynia should always be considered in the differential diagnosis. The history should document the nature of the pain, onset, severity, and effect on everyday life and/or sexual function. In addition to dyspareunia and pain provoked by any local contact, women with provoked vulvodynia may report constant or intermittent spontaneous discomfort such burning, aching, rawness, or irritation [28]. Vaginal symptoms including discharge, bleeding, should be sought. Nongenital menopausal symptoms, all medications, and all vulvar contacts (soaps, detergents, over-the-counter products) should be reviewed. Recent research has shown increasing evidence for comorbidity of vulvodynia and other chronic pain conditions such as fibromyalgia, interstitial cystitis, temporomandibular joint and muscle disorder (TMD), and irritable bowel syndrome [29] (Figs. 21.1 and 21.2). As such, patients with vulvar pain should be asked about symptoms related to these pain disorders as their presence may heighten the concern for vulvodynia.
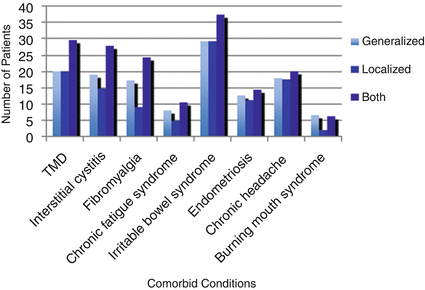

Fig. 21.1
Distribution of the eight comorbid conditions in each of the three types of vulvodynia (generalized, local, and both) in 1,457 women with self-reported vulvodynia. TMD temporomandibular joint, and muscle disorder (Source of data is Ref. [29])
A careful inspection of the vulva and vagina should be undertaken aiming at ruling out an inflammatory, infectious, or neoplastic cause of the pain and to sustain the diagnosis of provoked vulvodynia through the cotton swab test. For instance, the pain of provoked vestibulodynia is elicited by lightly touching the vestibule with a moistened cotton swab.
The presence of vulvar lesions does not exclude the diagnosis of vulvodynia. For example, the presence of psoriasis or warts on one labium majus cannot be held responsible for a spontaneous diffuse chronic burning vulvar pain or for an introital dyspareunia. Indeed vulvodynia may be associated with other nonrelevant conditions. In addition, anatomic variants such as vestibular papillae, Fordyce’s granules, or vestibular erythema should not be misinterpreted as causes of vulvar pain. As opposed to abnormal vestibular erythemas, physiological vestibular erythemas are macular (not raised), focal (posterior part of the vestibule, particularly around the openings of the Bartholin’s gland), symmetrical, and have ill-defined borders.
Pelvic exams should include palpation of the levator muscles to assess muscle spasm, bladder, and urethra for tenderness and bimanual exam to exclude pelvic pathology. Neurologic exam includes a search for sphincter disturbances (urinary, anal) by history taking and search for objective neurologic abnormalities such as anesthesia or hypoesthesia and perineal reflex abolition such as the “anal wink;” that is, scratching the perianal area gently with the sharp end of a cotton swab and observing the contracture of the external anal sphincter.
In the absence of any visible vulvar lesions or skin changes, a biopsy is not indicated and would not aid in the diagnosis of vulvodynia. In a menopausal woman, vulvovaginal atrophy is one of the possible causes of provoked vulvar pain, and signs such as loss of pubic hair, labial flattening or fusion, loss of vaginal rugae, or an elevated pH should be documented. In VVA, a vaginal wet prep would show an increase in vaginal parabasal cells and white blood cells without evidence of any pathogens such as yeast or bacterial vaginosis. Addition of a drop of Wright’s stain to the wet prep will more clearly define the presence of parabasal cells. Vaginal cultures should be sent if evidence of infection is present. Vaginal specimens should be systematically taken to look for infection either responsible for or associated with the pain.
In menopausal women complaining from dyspareunia, a trial of topical estrogen (in the absence of contraindications) should be the first-line treatment especially if there is evidence of VVA on exam. If symptoms do not resolve with these measures, treatments for vulvodynia should be initiated. Spontaneous diffuse vulvar burning is not a manifestation of VVA and, in the absence of relevant findings, is more likely related to vulvodynia.
21.6 Treatment
There is no “one size fits all” treatment of vulvodynia. Experts agree that a multifaceted treatment is the best approach to this condition [30, 31]. Choices will depend on the patient, her partner, the local health-care system, and the costs. Vulvar care measures, pharmacological treatments (both topical and oral); physical therapy; and personal, couple, or sexual counseling, are all possible components of successful treatment.
The National Vulvodynia Association (NVA) is an excellent source for educational materials which actively promotes support for these patients (www.nva.org).
21.6.1 Vulvar Care Measures
Gentle genital hygiene is important to prevent irritation which may aggravate vulvodynia. The vulva should be washed with plain warm water only or with a gentle, unscented soap and then patted dry. Vulvar irritants such as bath salts or over-the-counter feminine hygiene products, such as douches, sprays, and scented wipes, should be avoided. Ice packs (easily made by freezing water in a 16 oz. plastic soda or water bottle) can be applied through clothing for temporary relief. Adequate lubrication during intercourse and vaginal moisturizers for noncoital lubrication should be encouraged [33] (Table 21.2).
Table 21.2
Treatment guidelines for vulvodynia in menopause
Comorbidity | Vulvovaginal atrophy should be adequately treated prior to and during vulvodynia treatment |
Multiple treatments | Only one treatment should be introduced at a time |
Dosage | Medications should be started at the lowest dose and titrated upwards slowly. In older women, a longer interval between advancing doses can be considered |
Documentation | Patient pain diaries and examinations with specific pain location and severity recorded in the patients chart will help document response and guide treatment |
Adjunct therapy | Physical therapy is a helpful adjunct to medical therapy |
Counseling options | Counseling should be offered (individual, couple, sexual) as appropriate |
Surgery | Surgery should be reserved for localized vestibulodynia only after failure of medical therapy and careful evaluation of risks and benefits |
21.6.2 Topical Medications
Lidocaine anesthetic ointment can be used both for symptomatic relief and to reduce coital pain in women with localized vestibulodynia. Lidocaine gel 5 % applied externally 10–20 min prior to intercourse aids in reducing pain with penetration with minimal effect on overall sexual sensation in many women. Direct application to the clitoris should be avoided. One study showed that daily applications of topical lidocaine were equally effective as biofeedback in relieving symptoms at 12 months [34], whereas in another double-blind, placebo-controlled study, lidocaine failed to improve vulvodynia symptoms more than placebo when applied four times a day for 12 weeks [35]. Many topical medications have been used anecdotally or shown to be effective in small studies or case series. These include 2–6 % gabapentin formulation [36], compounded topical estradiol 0.03 % with testosterone 0.1 % [37], capsaicin [38], amitriptyline 2 % cream (sometimes combined with baclofen 2 %) [39], or nifedipine cream [40]. None of these treatments are evidence based, and all of these treatments are off-label for the treatment of vulvodynia.
Intolerance to topical treatments is common. Although contact dermatitis has been documented [41], discomfort (mostly burning) following topical applications is related neither to an allergy nor to an irritation. Diluting topical preparations with tolerated substances, such as lubricating gels, estrogen creams, and mineral or vegetable oils, may be helpful.
21.6.3 Oral Medications
Oral medications for vulvodynia are aimed at the treatment of neurogenic pain. The most frequently prescribed treatment is the tricyclic antidepressants, amitriptyline, desipramine, or nortriptyline. The latter two have a more favorable side effect profile with less sedation and anticholinergic effects. Documentation of a normal electrocardiogram (ECG) is recommended by some in patients over the age of 50. Dosage is started low, usually at 10 mg at night and slowly titrated upward by 10 mg every 1–2 weeks until relief is achieved. Generally, if there is no relief at a dosage of 75 mg, then treatment is discontinued. Patients must not stop tricyclics abruptly.
Gabapentin has been shown in small studies to provide relief for vulvodynia with fewer side effects than the tricyclic antidepressants. Gabapentin 100 mg at bedtime increased by 100 mg every 2–7 days to a maximum of 3,600 mg/day in divided doses is one treatment regimen. Side effects include nausea and sedation. Gabapentin was put on the Food and Drug Administration (FDA) MedWatch list for rare reports of rhabdomyolysis (2011) [42]. Patients should be informed to call with any muscle aches or pains or new-onset dark urine for evaluation. A randomized controlled trial is currently ongoing [43].
Other medications used in the treatment of vulvodynia include pregabalin (titrated slowly to a maximal dose of 600 mg daily in divided doses), venlafaxine (37.5 mg initially to a maximum daily dose of 375 mg), and duloxetine (20–60 mg daily). None of these treatments have an evidence-based efficacy [44], and a high and potentially serious side effect profile limits their use.
21.6.4 Botox
Botulinum neurotoxin type A (Botox) injections into the vestibule seems to be a safe and effective treatment of provoked vestibulodynia [45, 46]. This treatment has been used in small numbers of women in two randomized trials. Botox is postulated to relieve both the muscular hyperactivity of the perineum and to reduce the pain through a blockage of the release of neuropeptides and neurotransmitters. Cost may prove to be a limiting factor of this treatment.
21.6.5 Physical Therapy
Patients with provoked vestibulodynia, compared to non-affected women, have pelvic floor muscle hypertonicity (i.e., increased tension) [47], which may exacerbate or propagate the condition. Pelvic floor physical therapy has been shown to provide vulvovaginal pain relief and improve sexual functioning. Patients report a physical, emotional, and sexual improvement with physical therapy, especially if it is part of a multifaceted treatment approach [48–50]. Pelvic floor physiotherapists utilize internal and external soft tissue mobilization and massage, release of trigger points, biofeedback, and postural exercises. Patients are provided with at-home exercises which help shorten the course of treatment. Medical professionals can provide instructional exams for patients, demonstrating muscle relaxation and contraction and/or advice vaginal dilators for women in addition to physical therapy or for those women who cannot or choose not to use physical therapy. No study has been carried out to evaluate the efficacy of physiotherapy in unprovoked vulvodynia. Spinal cord stimulation and transcutaneous electrical nerve stimulation are less frequently used to treat vulvodynia [51, 52].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree