Fig. 20.1
Vulval angiomata
20.3 Vulval Infection
One of the more common reasons for women between puberty and the menopause to have vulval symptoms is infection and in particular due to the yeast Candida albicans. This occurs during pregnancy, while using oral contraceptives and following prolonged courses of broad-spectrum antibiotics.
It is commonly stated that the postmenopausal vulva is more vulnerable to infection, but on the basis of alterations within the vagina. Studies using swab and culture methods are now being replaced by molecular techniques for ‘microbial communities’, but little is available for description of what happens after the menopause [28].
It is most unlikely that postmenopausal women will have symptoms, e.g. chronic itch, vaginal discharge and dyspareunia due to vaginal yeasts, unless they are taking topical or systemic estrogen therapy [29] or are diabetic (Fig. 20.2). Before ascribing vulval symptoms in any postmenopausal women to yeasts, a low vaginal swab should be taken (by the woman when she is symptomatic or by her doctor) and examined for confirmation. Symptoms thought to be due to yeasts but associated with a negative swab require closer examination (as per the next section). Those women who have confirmed yeasts and are not on estrogen therapy should be screened/tested for diabetes mellitus.


Fig. 20.2
Maceration due to Candida albicans in a poorly controlled diabetic
The reasons for younger women being more susceptible to Candida species remain unclear, but include promotional changes in pH, direct effect of estrogens on adherence of yeasts to vaginal mucosa, effects of menstruation and tampon/menstrual pad use on vaginal microflora [30], an estrogen effect on vaginal immunity [31] and the availability of antifungal therapies as over-the-counter sales without an examination or prescription from their doctor [32].
The impact of bacterial vaginosis and chlamydial or gonococcal bartholinitis is less likely in postmenopausal women. Viral changes associated with human papilloma virus will be discussed later. Herpes simplex lesions may still occur or reactivate in postmenopausal women. Rarely herpes zoster, due to varicella-zoster virus, can be seen involving part of the vulval area, i.e. unilateral involvement of thoracolumbar T12/L1 or sacral S2, S3 or S4 dermatomes (Fig. 20.3). Molluscum contagiosum has been seen in immune-competent postmenopausal women although more commonly seen in young adults (Fig. 20.4).
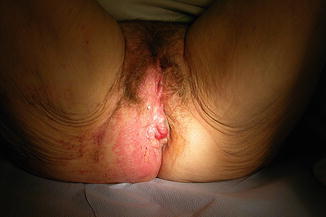

Fig. 20.3
Herpes zoster unilaterally involving the perineum and buttock

Fig. 20.4
Molluscum contagiosum
Superficial skin infections due to staphylococcal or streptococcal species are less frequently seen than in premenopausal women (partly associated with hair removal by waxing or shaving), but may follow skin damage from urinary or faecal incontinence (see section below on vulval dermatitis).
Deeper infections, e.g. Hidradenitis suppurativa (Fig. 20.5), do occur in perimenopausal women but usually settle after the menopause [33]. Skin infection complicating vulval surgery can complicate healing in any age group, particularly if obese, diabetic or immune-compromised. Necrotizing fasciitis or Fournier’s gangrene is reported but extremely uncommon [34].
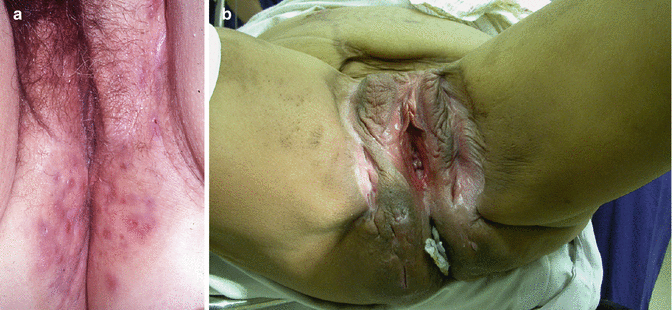

Fig. 20.5
Hidradenitis suppurativa. (a) Changes in the perianal area. (b) More extensive and with previous surgical changes
20.4 Vulval Patients
The majority of patients who attend a gynaecology, dermatology or genitourinary clinic do so because they are symptomatic. For most, they will complain of itch or scratching (pruritus), while a minority will have pain, burning, soreness or rawness. Some will attend because of splitting, discomfort or sexual difficulty. However, some patients will be referred because abnormal, unusual or altered appearances will be noted when the patient attends for something else, e.g. to have a smear taken, or a gynaecological examination for other reasons.
While clinical appearances may allow the medical or nursing practitioner to make a diagnosis, more frequently it will require an appropriate history and to use correct examination techniques and biopsy procedures. History should include past history, e.g. flexural eczema or asthma, and family history, e.g. psoriasis and LS both having familial clustering. Other autoimmune disorders may be relevant. Careful documentation of all treatments previously used including topical applications is important to recognise that the appearances may have been modified, to avoid prescribing something that has not worked previously, and to identify possible contact allergens or irritants including soaps, perfumes, preservatives and components of sanitary pads.
Examination should include the fingernails (Fig. 20.6) and skin of the hands, wrists, elbows, scalp and face; the oral cavity (Fig. 20.7), gums and tongue; the trunk and the lower limbs. Examination of the vulva requires exposure of the pudendum from pubes and inguinal areas to the natal cleft and cannot be done easily with the patient lying flat on a couch; the dorsal position gives some viewing but the patient then needs turning on her side, sometimes with straightening of the upper leg, to get complete viewing. Adequate lighting is paramount. Colposcopy is not essential, but a colposcopy chair provides good positioning, and the associated light of the colposcope allows viewing without the examiner’s head or hair obscuring the light source. Areas of colour change are noted and examined at higher magnification (a hand-held looking glass is an alternative to a colposcope and may now come with an in-built light source) for change in surface contour – exophytic or ulcerative. Red areas may represent altered capillary dilatation, but angiogenesis should be considered. White areas with abnormally thickened keratin may be the result of scratch damage but may represent more significant pathology.
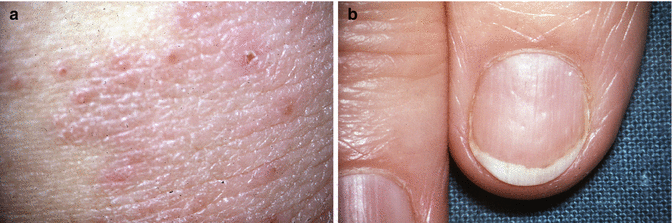
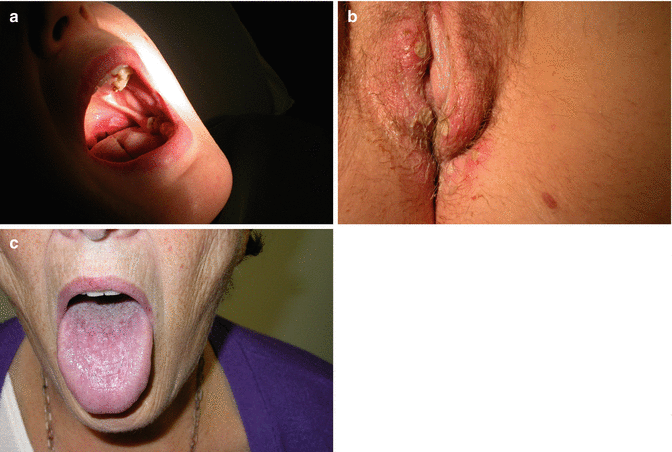

Fig. 20.6
(a) Vulval psoriasis and (b) nails showing pitting

Fig. 20.7
(a) Pharyngeal and (b) vulval ulceration with Behcet’s disease. (c) Appearance of tongue with oral lichen planus
Applications of 5 % acetic acid or 1 % toluidine blue solutions are not performed for every patient but certainly for anyone who has previously had an abnormal smear. Acetic acid penetrates mucosa quickly but not keratinised skin; it precipitates nucleoprotein so that neoplastic areas become visible (as for colposcopy of the cervix [35]), but it will take longer, the acetowhite epithelium may not be as obvious as on the cervix, and little information can be obtained if scratching has produced thickening of the keratin (hyperkeratosis or lichenification). Acetowhite epithelium does not equate to VIN or to HPV presence [36], and biopsy is required of the area. Toluidine blue stains the nuclei of cells (as haematoxylin does in histopathology). Surface squames consist mainly of cytoplasm with small pyknotic nuclei and therefore take up minimal blue staining. Where squamous differentiation and maturation is abnormal (as for VIN, usual type) the nuclei are larger, or if the surface cells have been scratched or scraped away, blue areas of staining will be defined, and these should be biopsied.
Biopsy is not necessary for every diagnosis. Some lesions, e.g. dermatitis/eczema, will be characteristic in their distribution, e.g. interlabial folds or perianal skin. Other lesions may have non-specific appearances as described above, and biopsy will be the only way to characterise the lesion (see classification below). Local anaesthesia, e.g. 2 % lignocaine with adrenaline as a vasoconstrictor, is infiltrated via a 27-gauge needle adjacent to but not into the lesion, and the biopsy is taken with a 4 mm biopsy (e.g. Stiefel) punch. Haemostasis is obtained with silver nitrate or Monsel’s solution and pressure for a few minutes. If the patient is taking aspirin or warfarin, or multiple sites need to be biopsied to map out the extent of the lesion, e.g. Paget’s or VIN, the biopsies are better taken in the theatre, often with a short general anaesthetic and the biopsy sites undersewn with sutures for haemostasis [37].
20.4.1 Classification (ISSVD 2006) of Vulval Dermatoses
Spongiotic pattern: atopic dermatitis, allergic contact dermatitis, irritant contact dermatitis
Acanthotic pattern (formerly squamous cell hyperplasia): psoriasis, lichen simplex chronicus, primary (idiopathic), secondary (superimposed on LS, lichen planus or other vulval disease)
Lichenoid pattern: LS, lichen planus
Dermal homogenisation/sclerosis pattern: LS
Vesiculobullous pattern: pemphigoid (cicatricial type), linear immunoglobulin A disease
Acantholytic pattern: Hailey–Hailey disease, Darier’s disease, papular genitocrural acantholysis
Granulomatous pattern: Crohn’s disease, Melkersson–Rosenthal syndrome
Vasculopathic pattern: aphthous ulcers, Behcet’s disease, plasma cell vulvitis [38].
20.5 Vulval Dermatitis/Eczema/Lichenification/Lichen Simplex Chronicus/Contact and Irritant Dermatitis
Contact dermatitis occurs as an allergic response to various allergens including topical antibiotics, anaesthetic and antihistamine creams, deodorants and perfumes (e.g. Balsam of Peru as used in various haemorrhoid creams), lanolin, azodyes in nylons, biological washing powders, etc. An increasing number of referrals to vulval clinics are being seen because of the current trend to remove vulval hair. Some women have applied depilatory creams or waxes with subsequent irritant reaction, while others have inflicted skin damage by shaving.
An increasing challenge in the elderly is control of bladder and bowel function. Sometimes the vulval skin will be irritated by the constant wearing of pads or incontinence devices, but also by the effect of urine or faecal material in contact with the skin. Such events do not necessarily relate to the menopause, although researchers have highlighted the role estrogen plays in maintaining function of the lower urinary tract which in turn preserves urinary continence. As well as the genital tract, the urethra, bladder and pelvic floor also have estrogen receptors. Postmenopausal low levels of estrogen lead to urogenital atrophy and are associated with lower urinary tract symptoms, such as frequency, urgency, incontinence and nocturia [39]. The diminished action of estrogen reduces sensitivity of the urethral smooth muscle, which predisposes to incontinence. The prevalence of urge incontinence, in particular, increases with the menopause [40]. Elderly women have increased susceptibility to the so-termed incontinence dermatitis when the perineal skin is subject to urinary moisture under occlusion. The skin becomes inflamed and its barrier function is damaged by friction, rise in pH and faecal enzymes; this damage is perpetuated by chronic incontinence [41].
The clinical appearance of dermatitis (Fig. 20.8) is diffuse but sometimes focal erythema and oedema with superimposed infection or lichenification (Fig. 20.9). The terms ‘lichen’ and ‘lichenification’ cause confusion because of their similarity. Lichenification describes the patchwork covering of the rocks of the West of Scotland (and elsewhere) and is best illustrated by the appearance of the skin over the elbows and knuckles (metacarpophalangeal joints) where the shin is thickened and patterned unlike skin elsewhere. Biopsy is usually not required unless there is no response to treatment; then other diagnoses should be considered.
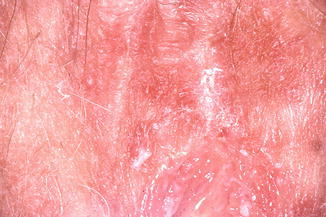
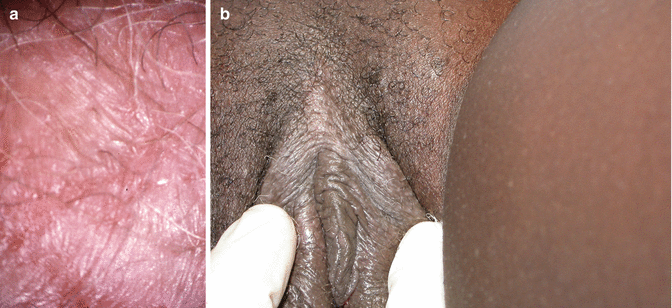

Fig. 20.8
Dermatitis caused by perfumed soap

Fig. 20.9
(a, b) The appearance of lichenification
Patch testing may identify the allergen to allow removal or avoidance of the factor, and moisturising cream or mild steroids should provide local control. Haverhoek et al. [42] reported that 81 % of the patients that they saw with vulval pruritus had at least one contact allergen detected on patch `testing.
Some topical preparations will cause irritation over time (i.e. in a chronic fashion). The vulval appearances will be less obvious, with minimal erythema but with pallor or pigmentation and with dryness, thickening and cracking – lichenification or lichen simplex chronicus. Lichen simplex chronicus (previously known as ‘neurodermatitis’) occurs in normal skin which becomes thick and fissured in response to the trauma of constant scratching. These lesions are not usually symmetrical and occur in vulval areas accessible to scratching.
Treatment consists of the use of emollients or topical corticosteroids of low-to-moderate potency. Sometimes, sedation at night is useful to stop nocturnal scratching. Once control is gained, assessment for an underlying cause or lesion is often necessary.
20.6 Lichen Sclerosus
Lichen sclerosus represents approximately one quarter of the patients attending our vulval clinic, and the majority of them will be postmenopausal [43]. It is found once in every 800 prepubertal girls [44] and increases with age to be reported as frequent as 1 in 30 elderly women in residential care [45]. It is an inflammatory dermatosis and is frequently symptomatic, causing pruritus, sleeplessness, dyspareunia and constipation. It may produce major architectural changes and alterations in vulval appearance and function and has a small but important risk of cancer; in the UK 60–70 % of vulval squamous cell carcinomas occur against a background of LS [46].
Lichen sclerosus involves the pudendum either partially or completely as a ‘figure of eight’, encircling the vulva and anus and altering the clitoris and its prepuce, the labia minora and inner aspects of the labia majora and the fourchette and perineum. It is usually symmetrical, bilateral and central, but may involve more lateral structures including the genitofemoral creases and the natal cleft. It involves the skin, but does not involve the mucosa of the vestibule, vagina or anus (although ‘overlap’ with erosive lichen planus is recognised). The lesions consist of thin, pearly, ivory or porcelain white crinkly plaques. Sometimes there is shrinkage and absorption of the labia minora, but sometimes they coapt anteriorly to cause clitoral phimosis and narrowing of the introitus to make intercourse impossible and to impede micturition. Scratching may result in epidermal thickening (lichenification) but sometimes erosion and superficial ulceration (the previous term used by clinicians and endorsed by the ISSVD was ‘lichen sclerosus et atrophicus’). Areas of ecchymoses and later haemosiderin pigmentation are common. LS may involve the trunk and limbs in up to 20 % of patients [47] (Fig. 20.10).
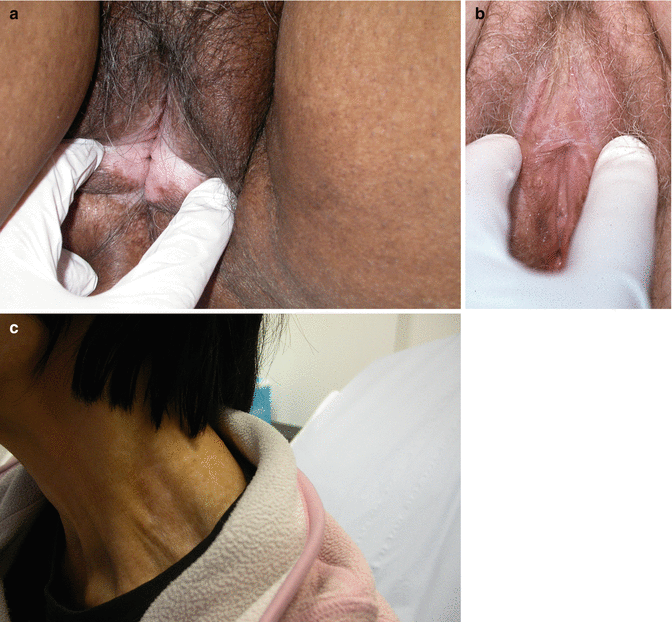

Fig. 20.10
(a) Lichen sclerosus. (b) Lichen sclerosus with phimosis. (c) Extragenital lichen sclerosus, involving the neck
The histological features of LS include epidermal atrophy, dermal oedema, hyalinisation of the superficial dermal collagen and subdermal chronic inflammatory infiltrate. However, occasionally the clinical features will resemble typical LS, and yet the biopsy shows minimal features. Sometimes the terms ‘early’ and ‘late’ are used to indicate the level of the inflammatory process, and these must not be misinterpreted as recent compared to longer duration.
The cause(s) of LS must consider the increased frequency in women (c.f., men) and with increasing age. It may show clustering within families, and a link with HLA DQ7 has been reported [48]. The association with autoimmunity has been widely reported. In the last decade Howard et al. [49] have found circulating basement membrane zone antibody in the serum of patients with LS, and Oyama et al. [50] describe an antibody to extracellular matrix protein 1. Farrell et al. [51] described the presence of interferon-gamma and other inflammatory cytokines within lesional epidermis, underlying dermis and within the inflammatory areas.
Explanation is required why LS is frequently seen in the UK, Europe, and especially Scandinavia, North and South America, Oceania and Southern Africa and yet is infrequent in Northern Africa, Arabia, Pakistan, India, Malaysia, Thailand, Singapore, Philippines and Hong Kong. The authors’ observations are offset by data that a quarter of the women attending vulval clinic were born outside the UK, including countries within the areas listed above. It is rare to diagnose LS in women newly arrived in London, but prolonged domicile may be important. The authors are currently researching the possible role of vitamin D deficiency in causing LS. Vitamin D is generated from sun exposure to the head, arms and body and from oily fish and egg yolks within the diet. Data gathered to date include many older women do not leave the UK for holidays in the sun, and avoid sun exposure or use high factor sun block (some of the patients have developed skin cancers in exposed areas and have been warned by their dermatologists to keep out of the sun, plus our latitude of 56 north; pigmented skin in some patients and more protective clothing in some ethnic and religious groups reduce the effect of the sun in producing vitamin D in the skin); avoid eggs because of their concurrent cardiovascular morbidity and therapy to lower cholesterol levels; and have little enthusiasm for eating certain fish (or are vegetarian). A few women take cod liver oil or vitamin supplements, e.g. with calcium, to protect their bones as the current literature discourages the use of hormone replacement therapy. Serum levels of vitamin D are abnormally low in some of our patients, but we are comparing these with control patients because many older people in London, including those who arrive from other countries, may have chronically low vitamin D levels. Certainly vitamin D deficiency, with rickets and osteomalacia, is being reported from British studies. Vitamin D deficiency has been linked with autoimmune diseases and certain cancers. Dietary supplementation may become an easier option than sending every postmenopausal woman for a holiday in the sun.
The treatment of LS usually requires the super-potent topical corticosteroid clobetasol propionate and following the protocols described by the British Association of Dermatologists [52]. A fingertip or ‘pea-sized’ amount of clobetasol ointment (Dermovate) is applied at night for 4 weeks, alternate nights for 4 weeks and then twice a week for 4 weeks before review. The pharmacokinetics of clobetasol suggests one application per 24 h is adequate. Many patients require reassurance that the potent steroid will not damage their skin when used according to instructions and to use applications on a regular basis because stopping treatment will frequently cause a rebound of symptoms. A 30 g tube should last for at least 12 weeks, and most patients with ongoing symptoms require 30–60 g of clobetasol per year. Emollients are used during the day and a soap alternative while washing. Testosterone cream no longer has any role in treating women. Alternative treatment use must be done with careful vigilance because of the increased risk of malignancy in patients who do not respond to clobetasol.
20.6.1 The Relationship Between LS and Vulval Cancer
This relationship continues to be an area of debate, and the positive side of the argument has been presented elsewhere [46]. Several authors have reported a prevalence of carcinoma of 2.5–5 % in women who present with LS [47, 53, 54]. It is unlikely that the development of malignancy led to the appearance of LS, although many of these women denied having had symptoms for any duration.
Nevertheless, there are individual cases or series of cases that document carcinoma occurring some time after a clinical diagnosis of LS, in spite of the use of appropriate clobetasol therapy. Jones et al. [55] reported that women who develop cancer with LS are more likely to show clinical evidence of squamous hyperplasia and are more likely to have been symptomatic for 5 years or more [56]. Cooper et al. [57] reported that six women developed carcinoma in spite of treatment in their series. Renaud-Vilmer et al. [58] reported that among eight women who developed cancer, one had discontinued treatment 3 years earlier, and a second patient had used treatment irregularly.
In my own unpublished vulval clinic data up until 2010, there were 655 patients with a diagnosis of LS made at their first visit, and 19 of them had concurrent squamous cell carcinoma of the vulva (2.9 %) and 5 with recurrent carcinoma within residual areas of LS. In subsequent follow-up, five further patients developed carcinoma; the first after 2 years was probably inadequately applying her treatment, the second (and only premenopausal patient of these five) after 4 years was optimally treated but frequently was symptomatic, the third had her cancer recognised 8 years after starting treatment with steroid but then being changed to a calcineurin inhibitor (see below), the fourth after 10 years of good symptom control was changed to systemic steroid to manage her fibromyalgia and the fifth after 12 years of supervised treatment became symptomatic when the national supply of diflucortolone valerate (another super-potent steroid available in the UK) stopped temporarily.
There are concerns that certain treatments may increase the risk of cancer. There have been publications advocating the application of tacrolimus or pimecrolimus as alternatives to steroids, but following reports of apparently rapid progression to cancer, the US Food and Drug Administration released a warning advising caution with their use [59].
There is now increasing awareness of the appearance of histological changes within LS that seem to predispose to a risk of progression to carcinoma. Many authors have commented previously on the finding of basal cell atypia (e.g. Leibovitz et al. [60]), and the latest terminology for vulval intraepithelial neoplasia (VIN) includes differentiated VIN as the preferred term [61]. Initial response to this term was that it was only found with specimens of invasive cancer, but it is now an important biopsy finding for a patient who remains symptomatic without a diagnosis of cancer (see below).
Many vulval cancers which have LS in the adjacent skin have been studied for various molecular alterations including changes in tumour suppressor gene activity and increased expression of mutant p53 [62, 63] and allelic imbalance with loss of heterozygosity or allelic gain [64]. The authors’ group has identified a mutation in codon 136 of exon 5 for p53 found in the areas of invasion and also in the adjacent LS [65]. Several patients with LS plus hyperplasia have progressed to carcinoma, and it is suggested that immunohistochemical staining of increased expression of p53 and Ki-67 may be a useful predictor of progression before invasion occurs [66].
Other factors that might place patients at increased risk of developing cancer include blood group; we found that 72 % of our patients with LS plus cancer were blood group A, compared with 38 % in a control population [67]. Similar associations with blood group A and the risk of gastric cancer suggest that the anti A antibody might be protective, or a link with particular polymorphisms increases susceptibility, e.g. to Helicobacter infection for gastric cancer [68]. After all this time of speculation, could another spirochaete, e.g. Borrelia burgdorferi, be linked with the risk of developing vulval cancer (Fig. 20.11)?
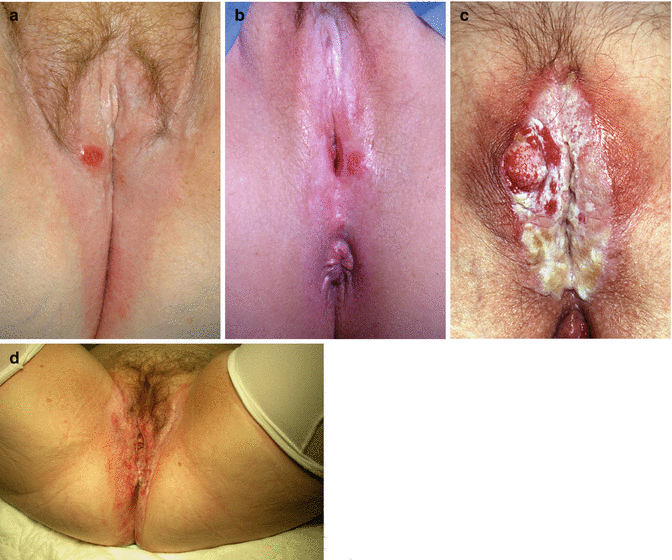

Fig. 20.11
(a–d) Examples of vulval cancer occurring in a background of lichen sclerosus
Recommendations for the specialist follow-up of women with LS:
1.
Women in whom difficulty exists with symptom control. This includes women who require potent topical corticosteroid application three or more times a week or greater than 30 g of ointment per 6 months for symptom control.
2.
Women previously treated for usual- or differentiated-type VIN and/or squamous cell carcinoma of the vulva. Differentiated VIN requires excision.
3.
Women with clinical evidence of localised skin thickening/hyperkeratosis require biopsies. If the skin thickening contains carcinoma or precancerous changes, or the thickening is unresponsive to topical steroids, they should be referred.
4.
Biopsies where the pathologist expresses ‘concern’ but cannot make a definite diagnosis of differentiated VIN should be referred. In this setting the clinician can choose between a limited period of intensive medical therapy with follow-up biopsies and excision at the outset [69].
The following mnemonic may be helpful when assessing LS:
L = labial changes (e.g. reduction or fusion)
I = introital changes (e.g. narrowing)
C = clitoral changes including phimosis
H = hyperkeratosis
E = elevated or eroded areas
N = neoplastic or suspicious area with hyperkeratosis or contour changes
S = symptom control
C = colour, usually with pallor but also ecchymoses and haemosiderin
L = localisation, usually central and symmetrical
E = extension posteriorly, causing anal symptoms and constipation
R = rest of the body; involvement elsewhere
O = other autoimmune conditions or associated symptoms
S = sexual difficulties
U = urinary difficulties
S = steroid use and misuse [70]
20.7 Lichen Planus
The lesions of lichen planus may be seen on mucous membranes or on cutaneous surfaces. Involvement of the vulva is usually with white patterned areas that are sometimes elevated and thickened (hypertrophic lichen planus) or may appear red and raw with features of erosion. Changes in the mouth are often seen. The vulval lesions may extend into the vagina where scarring, stenosis and adhesions make intercourse painful or impossible. In a small number of cases, ‘crossover’ from LS (involving the vulval skin) to lichen planus (involving the vestibule and vaginal mucosa) occurs (Fig. 20.12).
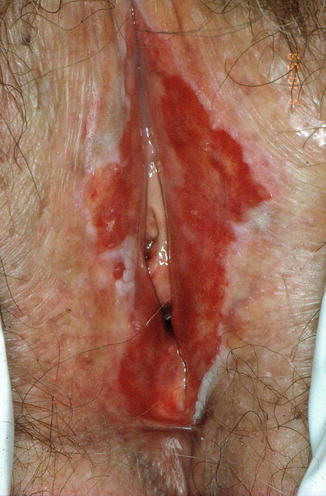

Fig. 20.12
Lichen planus with marked erosion of mucosal areas and loss of architecture
Histology will show liquefactive degeneration of the basal epidermal layer, long and pointed rete ridges, with parakeratosis, acanthosis and a dense dermal infiltrate of lymphocytes close to the dermal epidermal margin.
When the condition is severe, treatment can be difficult, requiring systemic steroids, azathioprine, hydroxychloroquine or other immune-modifying or chemotherapy agents such as methotrexate or mycophenolate mofetil. Lesser symptoms, particularly those externally on the vulva, can be managed with the application of topical corticosteroids; vaginal lesions can be managed with Colifoam (hydrocortisone) or Predfoam (prednisolone).
20.8 Vulval Intraepithelial Neoplasia
Although this term sounds closely similar to cervical intraepithelial neoplasia, there are some striking differences. Cervical neoplasia occurs in the transformation zone in association with squamous and columnar epithelium. The cervix has an epithelium where depth of changes can be judged: the vulva is the skin (epidermis) and, in addition to a basal layer which may be convoluted with deep rete ridges, also has a spinous or prickle cell layer, a granular layer and a superficial cornified or keratinised layer. Thus, dividing VIN into VIN 1, VIN 2 and VIN 3 is not easy or reproducible. While CIN 1 is a common biopsy finding and is part of a spectrum with possible progression to CIN 3, VIN 1 is uncommon and does not appear to have neoplastic potential.
The ISSVD Vulval Oncology Subcommittee has made recommendations about the terminology of VIN [61]. They argue that intraepithelial neoplasia of the vulva should be divided into two types: the first, known as VIN of usual (sometimes classic) type, encompasses what was previously VIN 2 and VIN 3 and is almost always associated with oncogenic HPV. These lesions may be pigmented (brown), white (hyperkeratotic) or red in appearance, and the histological features are described as warty, basaloid or mixed – the distinction may not have any prognostic significance, and warty and basaloid features may be seen concurrently in multifocal disease or seen over different examinations. The second is called differentiated VIN, usually seen in older women with LS or squamous cell hyperplasia and not associated with HPV (see above in association with LS) (Fig. 20.13).
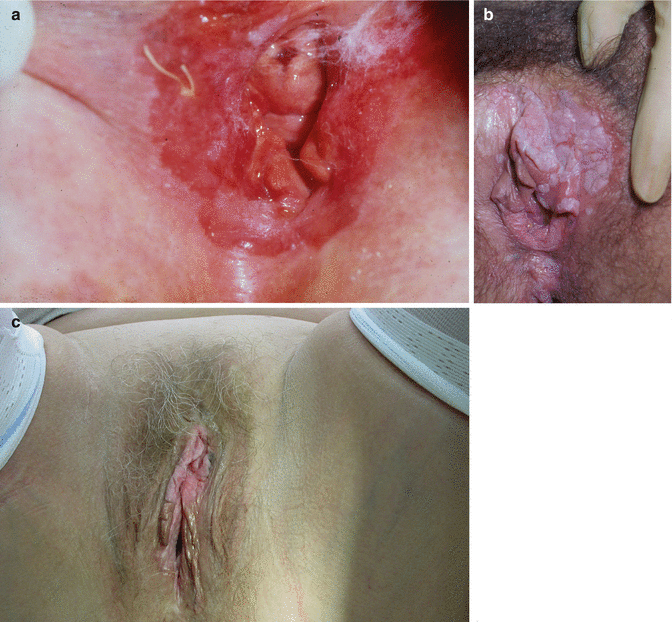

Fig. 20.13
(a–c) Examples of vulval intraepithelial neoplasia
Patients with CIN are usually identified by abnormal cervical cytology, whereas women with VIN present with symptoms, including itching, irritation, discomfort or splitting with intercourse in more than 80 %.
We know that when loop excision of the cervix is performed to treat CIN, less than 1 % of specimens will be found with microinvasive carcinoma. However, the chances of finding invasive cancer within the lesions of usual-type VIN range between 15 and 22 %, as summarised by MacLean [54] from data from five publications. Nugent et al. [73] have reported that postmenopausal women with VIN were more likely than premenopausal women to have related carcinoma. The difficulty with recognising invasion is partly because keratinised lesions will not show colposcopically visible features of vessel atypia/abnormality (angiogenesis) as it does for the cervix. The significance of invasion, even as little as 1 mm below the epidermo-dermal junction of the nearest dermal junction of the nearest papilla, is the increased chance of lymph node metastases; this risk only becomes significant with measurements of more than 3–5 mm from the nearest basement membrane of CIN.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





