30 Voiding Dysfunction and Urinary Retention
Voiding dysfunction or lower urinary tract dysfunction are terms used to describe various problems related to the bladder’s ability to store or empty urine. Urinary retention is the inability to complete the voiding phase of the micturition cycle and, often times, represents the end stage of voiding dysfunction. Physiologically, a problem may be present with either the bladder or the outlet, or both. Voiding dysfunction is manifest clinically in lower urinary tract symptoms (LUTS), which may be characterized as storage symptoms (frequency, urgency, nocturia, and urge incontinence) or emptying symptoms (decreased force of stream, incomplete emptying, hesitancy, straining to void, and urinary retention). Symptoms do not always correlate with the underlying pathology, and numerous conditions may exist that can have similar presentations. Thus, an often challenging task is to determine the specific etiology involved. In many cases, voiding dysfunction can be explained by neurologic disease, learned behavioral patterns, congenital, acquired, or by iatrogenic bladder outlet obstruction. In this chapter, we discuss normal voiding and the pathophysiologic events that lead to abnormal voiding as well as the evaluation, management, and treatment of women with specific types of voiding problems.
NEUROPHYSIOLOGY OF MICTURITION
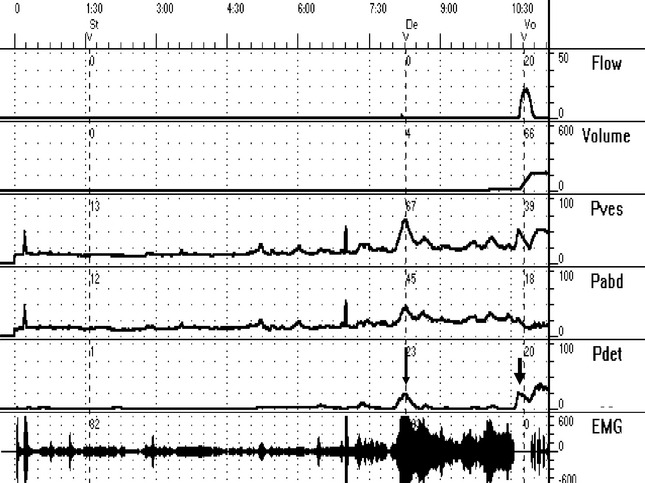
Normal voiding is accomplished by activation of the “micturition reflex” (Fig. 30-1). This is a coordinated event characterized by relaxation of the striated urethral sphincter, contraction of the detrusor, opening of the vesical neck and urethra, and onset of urine flow (Fig. 30-2). This reflex is integrated in the pontine micturition center, which is located in the rostral brainstem. Also, a sacral micturition center is located at S2–S4, through which the bladder can contract independently of cortical and pontine input. However, the pontine micturition center, through its neural pathways to the sacral micturition center, is responsible for coordinated and voluntary voiding. Both the autonomic and somatic nervous systems play a crucial role in lower urinary tract function. Normal voiding occurs when the bladder responds to threshold tension via its mechanoreceptors. To avoid a random occurrence, central nervous system inhibitory and facilitatory pathways are involved in coordination of urine storage and micturition.
Anatomically, the two areas of the spinal cord responsible for transmitting afferent input from the lower urinary tract are the lateral spinothalamic tracts and the posterior columns. The lateral spinothalamic tracts contain ascending routes responsible for transmitting bladder sensation and triggering voiding. Proprioceptive sensory impulses generated in bladder muscle and periurethral striated muscle travel in the posterior columns. The pontine micturition center controls efferent input to the bladder and external sphincter via two separate regions. The medial region is responsible for motor input to the bladder detrusor muscle via the reticulospinal tracts to the sacral intermediolateral cell groups, which contain preganglionic parasympathetic neurons that form the motor supply of the bladder detrusor muscle. Electrical stimulation of this region results in a prompt decrease in pelvic floor electromyography (EMG) and urethral pressure followed by an increase in intravesical pressure (normal voiding). The lateral region has specific projections via the corticospinal tracts to the nucleus of Onuf in the sacral cord that contains motor neurons innervating the pelvic floor, including the urethral and anal sphincters. Electrical stimulation of this region results in a rapid increase in urethral pressure and pelvic floor EMG but little increase in intravesical pressure (normal storage).
Peripheral innervation to the bladder, internal, and external sphincters is provided by the pelvic, hypogastric, and pudendal nerves. Parasympathetic efferent input to the bladder arises from the intermediolateral cell column of S2–S4 and travels as preganglionic fibers via the pelvic nerve to the pelvic plexus located on both sides of the rectum, from which postganglionic fibers innervate the bladder. Efferent sympathetic nerves to the bladder and urethra arise from the intermediolateral cell column of T10–L2 as preganglionic fibers that travel to ganglia located in the superior hypogastric plexus, from which arises the hypogastric nerve that contains the postganglionic sympathetic efferents to the bladder and urethra. The pudendal nerve carries the somatic input from S2–S4 to the external sphincter. Injury to any one of these nerves or their branches can result in a partial or complete denervation to the involved end organ.
EVALUATION OF VOIDING DYSFUNCTION
A complete physical examination is the next part of the evaluation. Particular attention should be paid to a systematic examination of the vagina and pelvis. One should first inspect the mucosa for atrophic changes as well as signs of previous surgery. Also, the position of the urethra, bladder neck, and bladder can be observed at rest and with straining to determine the degree of anterior vaginal wall prolapse (urethral hypermobility and cystocele). We like to use the posterior blade of a small vaginal speculum to retract the posterior vaginal wall to view the anterior wall. A Q-tip test can be used to quantify urethral hypermobility. The patient should also be assessed for stress urinary incontinence (SUI). An assessment of the uterus should be done to determine its support and position. In cases of previous hysterectomy, vaginal apex support and position are important. Uterine or apical prolapse are potential causes of obstruction. After examination of the anterior vaginal wall is completed, the blade of the speculum is rotated and the posterior wall and apex are inspected. Similar to other forms of prolapse, posterior vaginal wall prolapse, including rectocele and posterior enterocele, can cause obstruction and voiding dysfunction. Bimanual examination is performed to determine the presence of pelvic masses, including fibroids, which may also cause or contribute to voiding dysfunction. In patients with LUTS, a neurourologic examination should also be performed. Attention should be paid to the sensory and motor functions of the sacral nerves, including anal sphincter tone, perineal sensation, bulbocavernosus and anal wink reflex, strength of lower extremities, and deep tendon reflexes (knee and ankle). A careful neurologic examination may help to confirm suspected disease or uncover an unknown lesion.
Urodynamic testing can be an important part of the evaluation of female voiding dysfunction. However, it must always be kept in mind that it is only part of the evaluation to be used in conjunction with the rest of the assessment. Urodynamic findings inconsistent with patient symptoms or events occurring during testing that are uncharacteristic for patients during normal activity outside of the urodynamics lab should be interpreted with caution. We always advocate the use of multichannel urodynamics with simultaneous measurement of vesical and abdominal pressure and “subtracted” detrusor pressure during filling and voiding (see Fig. 30-2). The filling phase (cystometry) evaluates the ability of the bladder to effectively store urine by assessing bladder stability, compliance, and capacity. Pressure-flow analysis during voiding assesses bladder contractility and bladder outlet resistance. EMG may be added to assess the pelvic floor musculature and external sphincter. We have found video urodynamics to be particularly helpful in confirming and localizing anatomic or functional obstruction. The addition of simultaneous fluoroscopy of the bladder outlet will often discover an obstruction that would not have been made on the basis of multichannel urodynamics alone. When video urodynamics are not available, radiographic evaluation may still be useful in evaluating incomplete emptying. A standing cystogram in the anterior–posterior, oblique, and lateral positions, with and without straining, assesses the degree of bladder and urethral prolapse and displacement or distortion of the bladder. A voiding cystourethrogram will examine the bladder, bladder neck, and urethra during voiding and is important in uncovering anatomic abnormalities.
CLASSIFICATION OF VOIDING DYSFUNCTION
Many complex classification systems for voiding dysfunction have been proposed in the past; however, they failed to be diagnostically and therapeutically oriented. We have found the functional classification system, proposed by Wein (1981) and reviewed in Chapter 5, to be the most useful. This simple and practical classification system can be easily applied to our diagnostic criteria (e.g., urodynamics). Of equal importance is that treatment options can be chosen based on the classification.
Similarly, the effects of these problems can be classified as:
SPECIFIC CAUSES OF VOIDING DYSFUNCTION: DIAGNOSIS AND MANAGEMENT
Treatment of voiding dysfunction is dictated by several factors. First and foremost is the potential danger to the upper urinary tract associated with high storage pressures or the inability to empty the bladder. Although this is less common in nonneurogenic voiding dysfunction, any evidence of upper tract decompensation (hydronephrosis, elevated blood urea nitrogen [BUN], and creatinine) should be given immediate attention. Second is the degree of bother of the patient’s symptoms. In many cases, empirical therapy is an appropriate first step for patients with symptoms of voiding dysfunction. In patients without neurologic disease whose primary complaints are storage symptoms, such as urge incontinence, and who demonstrate an ability to empty, a course of anticholinergic therapy and/or bladder training programs (with or without biofeedback) is effective in relieving overactive bladder symptoms. In cases in which the diagnosis is not clear, or when abnormal emptying and storage coexist, a more comprehensive workup is necessary. The same can be said when empirical therapy fails and for cases of voiding dysfunction associated with neurologic disease. In all such cases further investigation with urodynamic testing is usually indicated. In these instances, the specific cause for the voiding dysfunction will direct treatment.
Distinguishing neurogenic from nonneurogenic voiding dysfunction is important. The latter category is often caused by bladder outlet obstruction, and this may be functional, as in the case of dysfunctional voiding and primary bladder neck obstruction (PBNO), or anatomic, as in the case of pelvic floor prolapse or post-surgical obstruction. A complete list of causes for bladder outlet obstruction in women is shown in Table 30-1.
Table 30-1 Anatomic and Functional Causes of Bladder Outlet Obstruction in Women
| Anatomic Obstruction | Functional Obstruction |
|---|---|
E. Iatrogenic obstruction
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|