A hypersensitivity reaction is an exaggerated and pathologic response by the immune system to a self- or foreign antigen. Hypersensitivity reactions differ in the mediators involved, mechanisms, timing, and clinical manifestations; however, there may be similar morphologic appearances making clinical diagnosis a challenge. There are four types of hypersensitivity reactions: Type I is immediate, anaphylactic; mediated by IgE, histamine, leukotrienes; and includes urticaria and angioedema. Type II is cytotoxic; mediated by IgM or IgG, complement; and includes some drug reactions and transfusion reaction. Type III is mediated by IgG or IgM antibodies in immune complexes; includes serum sickness, arthus reaction, vasculitis, or systemic lupus erythematosus. Type IV is delayed/cell mediated; activates reactions with antigen-specific T cells, such as poison ivy, allergic contact dermatitis, tuberculin reaction.
The hypersensitivity reactions addressed in this chapter include vasculitis, urticaria and angioedema, erythema multiforme (EM), and erythema nodosum (EN). These reactions range in severity from mild to life-threatening and can be triggered by drugs, infectious agents, foods, environmental allergens, malignancies, autoimmune disorders, other systemic illness, or unknown etiologies.
VASCULITIS
Vasculitis is an inflammatory process which involves the walls of any size or type of vessel causing damage that results in tissue necrosis. Cutaneous vasculitis may be limited to the skin only, may be a primary cutaneous vasculitis with secondary systemic involvement; or a cutaneous manifestation of a systemic vasculitis. All categories of vessels can be affected, including small, medium-sized, and large vessels of the arterial and/or venous systems. Small vessels include arterioles, capillaries, and postcapillary venules that are found in the superficial and mid-dermis of the skin (Table 16-1). Medium-sized vessels refer to the main visceral arteries and veins, and the small arteries and veins within the deep dermis or subcutaneous tissue (Table 16-2). Large vessels are the aorta, its major branches and corresponding veins, and other named arteries such as pulmonary or temporal artery. Cutaneous involvement occurs almost always with vasculitis of small and medium-size vessels and therefore the large-vessel vasculitides will only be briefly mentioned in this chapter.
The clinical spectrum of vasculitis presents a diagnostic challenge even for the experienced dermatology or rheumatology provider. In an attempt to classify the vasculitides, several schemes have been proposed; historically the classification was based on vessel size. Other criteria have been developed based on clinical signs and symptoms, histopathology, historical data, and the presence or absence of internal organ involvement. Most recently, The International Chapel Hill Consensus Conference on the Nomenclature of Systemic Vasculitides (CHCC2012) updated their original work of 1994. This is neither a classification system nor a diagnostic system, but rather it defines the nomenclature that should be used for a specifically defined disease process (Figure 16-1). For example, if a vasculitis is caused by direct invasion of pathogens, then the vasculitis should be specified as such (i.e., rickettsial vasculitis or syphilitic aortitis) rather than “infectious vasculitis.” Although there are differences among these criteria, a shared defining feature of vasculitis is an inflammation of blood vessel walls at some time during the course of the disease.
Furthermore, CCHC has renamed the vasculitis previously known as cutaneous leukocytoclastic vasculitis and is now referred to as cutaneous small-vessel vasculitis (CSVV). By definition, CSVV has the histologic findings associated with leukocytoclastic vasculitis but the involvement is limited to the small blood vessels of the skin, and no other organ system is involved. This may also be referred to as single-organ vasculitis. The following section will focus on the CSVV that is more commonly seen in the primary care setting, the importance of screening for systemic organ involvement, differential diagnoses, diagnostics, and prompt management.
Pathophysiology
The etiology of vasculitis can be broadly labeled as infectious or noninfectious, while the pathogenesis is poorly understood. Possible mechanisms of vascular damage include the deposition of immune complexes (i.e., Henoch–Schönlein purpura), presence of autoantibodies, or cell-mediated granuloma formation (T-cell response). Each pathway results in endothelial cell activation, leading to vessel obstruction and ischemia of dependent tissue.
Immune complex vasculitis. When vasculitis is caused by a hypersensitivity to an antigen, immune complexes form and are deposited in the vessel walls, which in turn activate complement that attracts neutrophils. The classic histologic features demonstrate transmural infiltration in and around small blood vessels (postcapillary venules) by neutrophils showing fragmentation of nuclei (karyorrhexis or leukocytoclasia), fibrinoid necrosis of the vessel walls, extravasation of erythrocytes, and swelling, shrinkage, or sloughing of endothelial cells. The late phase of the disease will show thrombosis of the affected postcapillary venules. Occlusion and necrosis in arteries and veins also occur secondary to aberrant coagulation caused by a hypercoagulable state or vasculopathy.
The difference between vasculitis and vasculopathy is subtle but needs to be considered throughout the diagnostic process since there may be similar clinical features and divergent diagnoses and treatments. The primary process in vasculitis is an inflammatory cell infiltrate triggering the clotting cascade and thrombosis, and may be seen in the late stages of healing vasculitic lesions. In vasculopathy, the primary process is thrombosis, usually due to a hypercoagulable state. Inflammatory cells enter the vessel and vessel wall in order to reestablish the local circulation. Thus, vascular inflammation is seen late in this primarily thrombotic process and can be misinterpreted as vasculitis on biopsy. The presence of livedo reticularis on clinical examination is usually, but not always, more consistent with vasculopathy.
Antineutrophil cytoplasmic antibodies-associated vasculitis. Antineutrophil cytoplasmic antibodies (ANCAs) are autoantibodies directed against an antigen in the cytoplasm of neutrophils. ANCAs are often associated with some system vasculitides, referred to as ANCA-associated vasculitis affecting mixed small- and medium-sized vessels. Indirect immunofluorescence (IIF) produces two major staining patterns: cytoplasmic ANCA (cANCA) and perinuclear ANCA (pANCA), while ELISA will identify antibodies to specific antigens.
Small-Vessel Vasculidities |
NOMENCLATURE | DESCRIPTION |
ANCA – Associated Vasculitides Necrotizing vasculitis, few or no immune deposits. Associated with pANCA or cANCA. Involves small arteries, arterioles, capillaries, and venules, but may also affect medium arteries and veins. | |
Microscopic polyangiitis* | Necrotizing vasculitis, few or no immune deposits. Necrotizing arteritis in small and medium-sized arteries may be present, necrotizing glomerulonephritis very common, and pulmonary capillaritis often occurs |
Granulomatosis with polyangiitis* (Wegener’s) | Necrotizing granulomatous inflammation involving upper and lower respiratory tract; necrotizing vasculitis in small-to-medium-sized vessels. Necrotizing glomerulonephritis is common. |
Eosinophilic granulomatosis with polyangiitis* (Churg–Strauss syndrome) | Necrotizing vasculitis of small and medium-sized vessels, associated with asthma and eosinophilia. Necrotizing granulomatous inflammation involves the respiratory tract. If glomerulonephritis is present, then usually ANCA is positive. |
Immune Complex Vasculitides* Notable deposits of immunoglobulin and/or complement of the vessel walls. Frequent glomerulonephritis. Involves small arteries, arterioles, capillaries, and venules. | |
Antiglomerular basement membrane disease | Involving glomerular and/or pulmonary capillaries with deposition of anti-GBM autoantibodies Inflammation can result in necrotizing glomerulonephritis or pulmonary hemorrhage. |
Cryoglobulinemic vasculitis | Cryoglobulin immune deposits associated with serum cryoglobulins Cutaneous, glomeruli, peripheral nerves often involved. |
IgA vasculitis | IgA1-dominant immune deposits Involve skin and gastrointestinal tract, frequently causing arthritis Glomerulonephritis indistinguishable from IgA nephropathy Onset often associated with URI or GI infection. Example: HSP |
Hypocomplementemic urticarial vasculitis | Presents with urticaria and hypocomplementemia. Associated with anti-C1q antibodies, glomerulonephritis, arthritis, COPD, and ocular inflammation |
Cutaneous leukocytoclastic vasculitis† (LCV) | Isolated cutaneous small-vessel vasculitis/leukocytoclastic vasculitis without systemic vasculitis or glomerulonephritis Usually limited to one organ |
*Some may also be categorized as vasculitis associated with systemic or unknown etiologies.
†LCV, traditionally classified as a single-organ vasculitis according to CHCC.
Adapted from Jennett, J. C., Falk, R.J., Bacon, P.A., Basu, N., Cid, M.C., Ferrario, F… . Watts, R. A. (2013). 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis & Rheumatism, 65(1), 1–11.
Many small-vessel vasculitides, particularly CSVV, have little or no vascular immune deposits and are idiopathic. There are many precipitating agents associated with vasculitis, such as chronic disease, malignancy, infections, or drugs (Table 16-3). It is worthwhile mentioning the occurrence of cocaine-associated vasculitis. Dermatologists, urgent care providers, emergency physicians, and primary care providers should be able to recognize the vasculitic lesions associated with the intravenous or intranasal use of cocaine. Characteristically, there is purpura and necrosis of the ears (Figure 16-2), nose, cheeks, and various other locations including the trunk. Levamisole, a drug contaminate in cocaine, is the suspected culprit, although a direct causal relationship has not been established.
Clinical Presentation
The importance of a detailed physical examination that accurately identifies the morphology of the presenting lesions cannot be overemphasized. Clinical presentation is critical in developing a differential diagnoses for vasculitis. It is absolutely essential that clinicians evaluate the patient for systemic involvement, which may present as fever, cough, myalgia, hemoptysis, abdominal pain, melena, weight loss, nausea/vomiting, diarrhea, hypertension, headache, visual disturbances, and renal insufficiency (urine sediment with protein and red and white cells).
The morphology of the cutaneous findings in any vasculitis will depend on the size of the vessels primarily affected and will provide clues to the type of vasculitis. Clinicians should understand the terminology frequently used in discussing these dermatoses (Table 16-4). Small-vessel vasculitis (SVV) results in petechia, purpura, erythematous macules, papules, and pustules (Figure 16-3). Palpable purpura occurs when vasculitis is present in postcapillary venules and is the hallmark of cutaneous vasculitis (Figures 16-4 to 16-6). Lesions typically evolve within 7 to 10 days of a causative exposure, and erupt in crops lasting about 1 to 4 weeks. The size of SVV lesions can range from 1 mm to several centimeters and may be asymptomatic, itchy, or painful. Edema may develop if nephritis is present. Painful nodules, pustules, and necrotic ulcers can present in specific types of SVV like granulomatosis with polyangiitis (GPA). The unusual distribution of lesions in GPA found in the oral and nasal mucosa should heighten the clinician’s index of suspicion. Yet most SVV lesions favor dependent sites such as legs and feet, or areas affected by trauma and tight-fitting clothing. The buttocks or back of a bedridden patient may be involved and reflects the influence of venous pressure due to gravity.
Medium- and Large-Vessel Vasculitides (Other Than Small Vessel) |
NOMENCLATURE | DESCRIPTION |
Large-Vessel Vasculitis Large arteries and aorta | |
Takayasu arteritis | Arteritis, often granulomatous, usually patients <50 years |
Giant cell arteritis | Arteritis, often granulomatous, usually patients >50 years. Predominantly the carotid and vertebral arteries; most commonly the temporal artery |
Medium-Vessel Vasculitis Any size artery, especially medium-sized artery like main visceral arteries & branches | |
Polyarteritis nodosa | Necrotizing arteritis without glomerulonephritis (of medium-sized or small arteries), or vasculitis not associated with ANCAs (arterioles, capillaries, or venules) |
Kawasaki disease | Arteritis associated with mucocutaneous lymph node syndrome (small, medium-sized, large arteries, and aorta) Coronary artery involvement usually seen in young children and infants |
Variable Vessel Vasculitis No predominant size or type of vessel | |
Behçet disease, Cogan syndrome | Primary categories of vasculitis because of the frequency of vasculitis in these conditions |
Single-Organ Vasculitis Any size artery and in a single organ | |
Named according to the affected organ or vessel | Distribution may be unifocal or diffuse within an organ/system Examples: cutaneous small-vessel vasculitis; testicular vasculitis; or central nervous system vasculitis |
Vasculitis Associated with Systemic Disease Caused by or associated with a systemic disease | |
Named according to disease-related etiology | Sometimes considered secondary vasculitis Examples: rheumatoid vasculitis; lupus vasculitis; or sarcoidosis vasculitis |
Vasculitis Associated with Probable Etiology Based on specific etiology | |
Named according to etiology | Sometimes considered a secondary vasculitis Examples: Hydralazine-associated microscopic polyangiitis; hepatitis B virus-associated vasculitis; syphilis-associated aortitis; or cancer-associated vasculitis |
Medium-vessel vasculitis occurs predominately in medium-size and small arteries (i.e., visceral arteries and branches). Polyarteritis nodosa (PAN) is a necrotizing medium-vessel vasculitis presenting as nodules (similar to EN), palpable purpura, and vesicles/bullae. Livedo reticularis may progress to ulcerations usually located on the lower extremities. The lesions are painful and may be accompanied by edema. Patients with PAN often have systemic symptoms.
Kawasaki disease (KD) is also a vasculitis of medium-size arteries, including coronary arteries and aorta. Although it is rare and self-limiting, recognition of the clinical presentation is important to avoid cardiac sequelae. It is more common in childhood and is discussed under Childhood Vasculitis.
Large-vessel vasculitis involving the aorta and large arteries means that affected patients have systemic involvement and appear much sicker. Ulcerations and sometimes gangrenous digits are seen with large-vessel involvement. Giant cell arteritis (GCA) occurs in patients over 50 years, who report constitutional symptoms along with temporal artery tenderness, visual loss, polymyalgia rheumatica, headache, jaw claudication, and temporal enlargement with absent pulse is common. Clinicians should assess the patient for aortic involvement, including signs and symptoms of aneurysm. In contrast, Takayasu arteritis is more prevalent in females younger than 50 years and affects the aorta and major branches. Granulomatous inflammation affects any or all of the organs, resulting in significant morbidity and mortality.
DIFFERENTIAL DIAGNOSIS | Vasculitis |
MORPHOLOGIC PRESENTATION | DIFFERENTIAL DIAGNOSIS |
Palpable purpuric papules/plaques (raised) | Arthropod bite Erythema multiforme Morbilliform drug eruption with hemorrhage Infectious emboli Cellulitis |
Purpuric macules/patches (flat) | Hemorrhage Trauma, solar/actinic purpura, medication-related (e.g., aspirin), thrombocytopenia, viral exanthem, scurvy Thrombosis Hypercoagulable state, purpura fulminans (e.g., DIC or sepsis), heparin or warfarin necrosis, livedoid vasculopathy Emboli Cholesterol, cardiac, fat, air Inflammation Pigmented purpura/capillaritis |
Urticarial lesions | Urticaria Arthropod bites Papular urticaria Serum sickness–like reaction Erythema multiforme Viral exanthem Sweet syndrome Urticarial phase of bullous pemphigoid |
Ulcers | Venous or arterial disease Pyoderma gangrenosum Livedoid vasculopathy Hypercoagulable state Calciphylaxis Infections (e.g., bacterial, mycobacterial, fungal) |
Nodules | Panniculitis Superficial migratory thrombophlebitis Dermal neoplasms Infections (e.g., bacterial, mycobacterial, fungal) Systemic vasculitides |
Diagnostics
The general approach for any patient with vasculitis should be to rule out other diseases that may mimic vasculitis; look for any underlying or secondary cause (disease, infection, drug, or malignancy); perform diagnostics to confirm the presence of a vasculitis; and conduct additional tests to identify the type and severity of the vasculitis.
A detailed history, review of systems, and clinical presentation will be invaluable in guiding the clinician’s development of a differential diagnosis or suspicion for secondary causes (Table 16-5). The initial diagnostic test should be a punch or excisional biopsy, the gold standard for evaluation of vasculitis, which should be taken from the center of a new lesion (preferably 24–48 hours old), making sure to collect some of the subcutaneous tissue if evaluating deeper, larger vessels. The histopathologic features of the biopsy can aid in identifying the type of vasculitis as well as other features which may indicate other systemic disease (i.e., interface dermatitis suggestive of connective tissue disease). Additional punch biopsy for direct immunofluorescence (preserved in Michel’s medium) may be helpful in identifying the deposition of immunoglobulins and complements in the blood vessels (i.e., Henoch–Schönlein purpura [HSP]).
A complete blood count with differential should be part of the initial workup. Patients with a primary vasculitis rarely have leukocytosis or thrombocytopenia, which, if present, should prompt further evaluation for an underlying disease, malignancy, or infection. Eosinophilia is seen in Churg–Strauss syndrome. Anemia could signify underlying lupus erythematosus or malignancy. A C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are usually elevated but are nonspecific markers of systemic inflammation. Blood urea nitrogen, creatinine, and electrolyte abnormalities may reflect kidney involvement. Abnormal liver function tests may be associated with underlying liver disease or malignancy. A stool guaiac will help assess for vasculitis of the bowel in patients with abdominal pain. Urinalysis with microscopy that is positive for red blood cell casts is suggestive of glomerulonephritis, while proteinuria is common in lupus nephritis. A chest x-ray may be recommended if the patient’s review of symptoms or clinical presentation suggests pulmonary involvement and may reveal pulmonary infiltrates, nodules, patchy consolidation, pleural effusion, and cardiomegaly.
Management
Treatment of vasculitis should be based on type and severity, as well as the presence of organ involvement. Management of primary CSVV seen in primary care will be the focus of this section. Medium- and large-vessel vasculitis are beyond the scope of this text and should be managed by dermatologists or appropriate specialists.
Most primary CSVV is mild and self-limiting. If an offending antigen like a drug is identified, then removing the source is the obvious first step. Underlying infection or disease should be treated at the outset. Treatment of CSVV (without systemic involvement) should proceed with supportive care, including leg elevation, rest, and gentle compression of legs for comfort. However, tight restrictive clothing may aggravate susceptible skin regions and should be avoided. Pain and inflammation can be treated with nonsteroidal anti-inflammatory drugs (NSAIDs), and pruritus may be attenuated with antihistamines such as hydroxyzine or diphenhydramine. Class I topical corticosteroid (TCS) ointment may also be helpful for adult patients, most of whom will not require systemic measures.
Short-term oral corticosteroids (prednisone) may be considered in patients with chronic (active disease that persists for more than 4 weeks) or recurring CSVV if hemorrhagic blisters and ulcerations are present. The dose and duration of treatment are dependent on the severity. A dosage of 0.5 mg/kg/day can be prescribed for 1 to 2 weeks or until new lesions cease to erupt. A taper over 2 to 4 weeks may be necessary and usually meets with complete resolution. Chronic forms of CSVV, or those with more severe cutaneous disease, may require higher doses of prednisone dosed 1 mg/kg/day to control symptoms. Since long-term use of corticosteroids has the risk of significant side effects, transitioning the patient to a steroid-sparing agent (i.e., colchicine, dapsone) is important. Patients on dapsone need G6PD testing prior to initiating therapy and careful monitoring for hemolytic anemia during therapy. Pentoxifylline has been effective when the CSVV is resistant. Recalcitrant CSVV or SVV with systemic involvement often requires management by dermatology specialists with azathioprine, methotrexate, cyclophosphamide, intravenous immunoglobulin, mycophenolate mofetil, rituximab, or plasmapheresis.

FIG. 16-1. Diagram of vasculitis showing vessel size, morphology, and related conditions.
Cutaneous Vasculitis: Causes and Precipitating Agents |
CAUSE |
| AGENT |
Infection | Bacterial β-hemolytic Streptococcus group A Staphylococcus aureus Mycobacterium leprae Mycobacterium tuberculosis Viral Hepatitis A, B, C (including vaccines) HSV HIV | Parvovirus B19 Fungal Candida albicans Protozoan Plasmodium malariae Helminthic infections Schistosoma haematobium Schistosoma mansoni |
Chronic disease state | Systemic lupus erythematosis Sjogren syndrome Rheumatoid arthritis Inflammatory bowel disease | Behcet disease Cystic fibrosis Cryoglobulinemia Bowel bypass syndrome |
Drug exposure | Common Anti-TNF agents COX-2 inhibitors Granulocyte colony-stimulating factor Hydralazine Leukotriene inhibitors Minocycline NSAIDs Penicillins Quinolones Streptokinase Occasional ACE inhibitors Allopurinol β-blockers Coumarins Furosemide Interferons | Macrolide antibiotics Phenytoin Retinoids Sulfonylureas Thiazides Trimethoprim–sulfamethoxazole Vancomycin Rare Aspirin Atypical antipsychotics Cocaine/levamisole contaminated Gabapentin Insulin Metformin Rituximab Selective serotonin-reuptake inhibitors Vitamins |
Neoplasm | Lymphoproliferative disorders Hodgkin disease Mycosis fungoides Myeloproliferative disorders | Lymphosarcoma Multiple myeloma Solid organ tumors Adult T-cell leukemia |
Other | Chemicals Insecticides Petroleum products | Food stuff allergens Milk proteins Gluten |
HSV, herpes simplex virus; COX, cyclooxygenase; NSAIDs, nonsteroidal anti-inflammatory drugs; TNF, tumor necrosis factor.
Adapted from Chung, L., Kea, B., & Fiorentino, D. F. (2008). Cutaneous vasculitis. In J. L. Bolognia, J. L. Jorizzo, & R. P. Rapini (Eds.), Dermatology (2nd ed., pp. 347–367). Spain: Mosby Elsevier.

FIG. 16-2. Characteristic purpura associated with levamisole (common contaminate in cocaine) induced small-vessel vasculitis on the ear (A) and arms (B).
Terminology |
Purpura | Lesions caused by extravasated red blood cells into skin, appearing red-brown or purple, flat (macule or patch) |
Palpable purpura | Papules or plaques (raised) which are blanchable erythema or nonblanchable component of visible hemorrhage. Diascopy can help distinguish between the two (below). |
Petechiae | Small pinpoint (<4 mm) purpuric macules, initially bright red that turn brown or rust-colored, and can be seen in thrombocytopenic states, as shown in Figure 16-3 |
Livedo | Macular violaceous netlike patterned erythema of the skin; may indicate vasculopathy |
Retiform purpura | Angulated or branched configuration of purpura reflecting the vascular architecture in the skin; vasculopathy diagnosis should be favored |
Ecchymosis | Large (>1 cm) blue-purple areas of interstitial hemorrhage |
Infarct | Areas of cutaneous necrosis secondary to occlusion of blood vessels. Lesions are dusky red and gray irregular macules surrounded by pink hyperemia and eventually turning black. It can be present in vasculitis, vasculopathy, or embolic disorders. |
Diascopy | Application of pressure using a glass slide on a red lesion to distinguish vascular dilatation (erythema) which blanches compared to redness due to extravasated red blood cells (purpura), which does not blanch. |
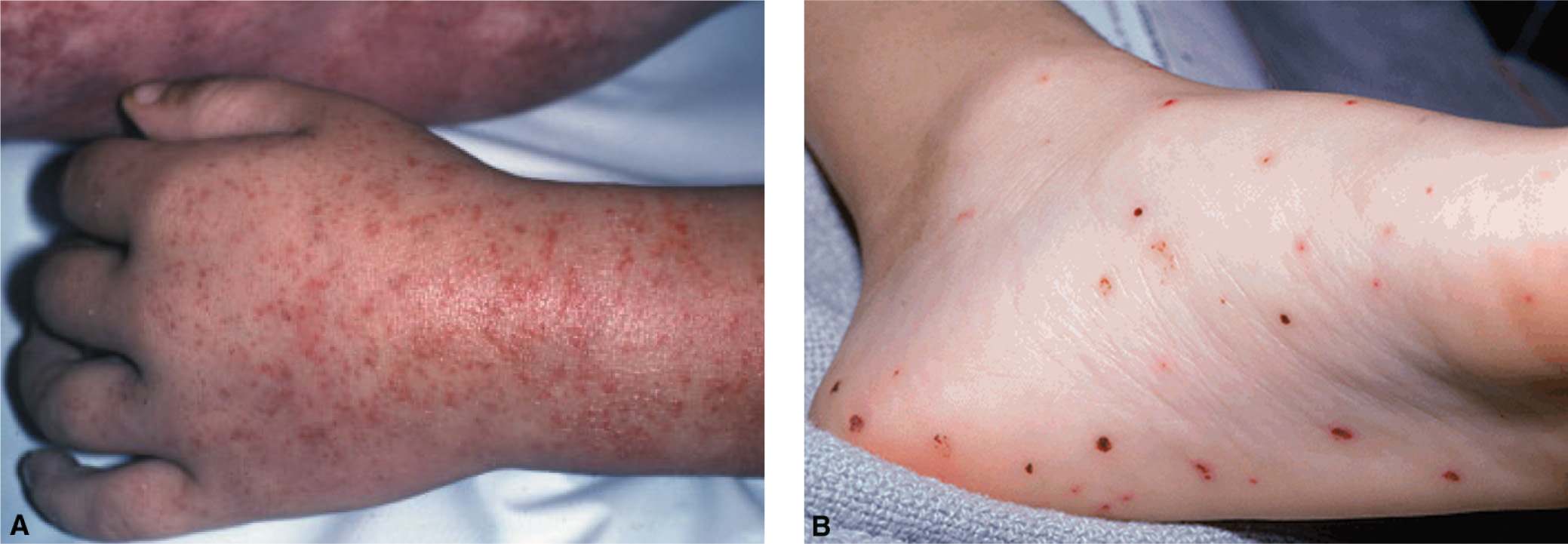
FIG. 16-3. A: Tiny skin hemorrhages (petechiae) in a child with thrombocytopenia. B: Petechiae on the foot and sole of a child with HSP with palpable purpura.
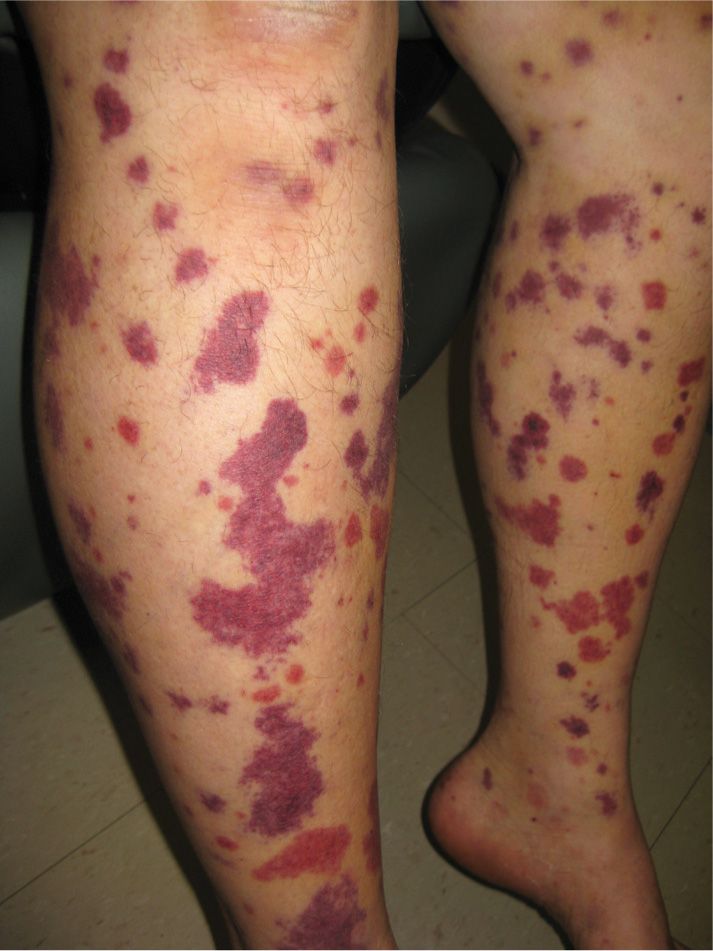
FIG. 16-4. Purpura in a patient with small-vessel vasculitis on the lower legs.
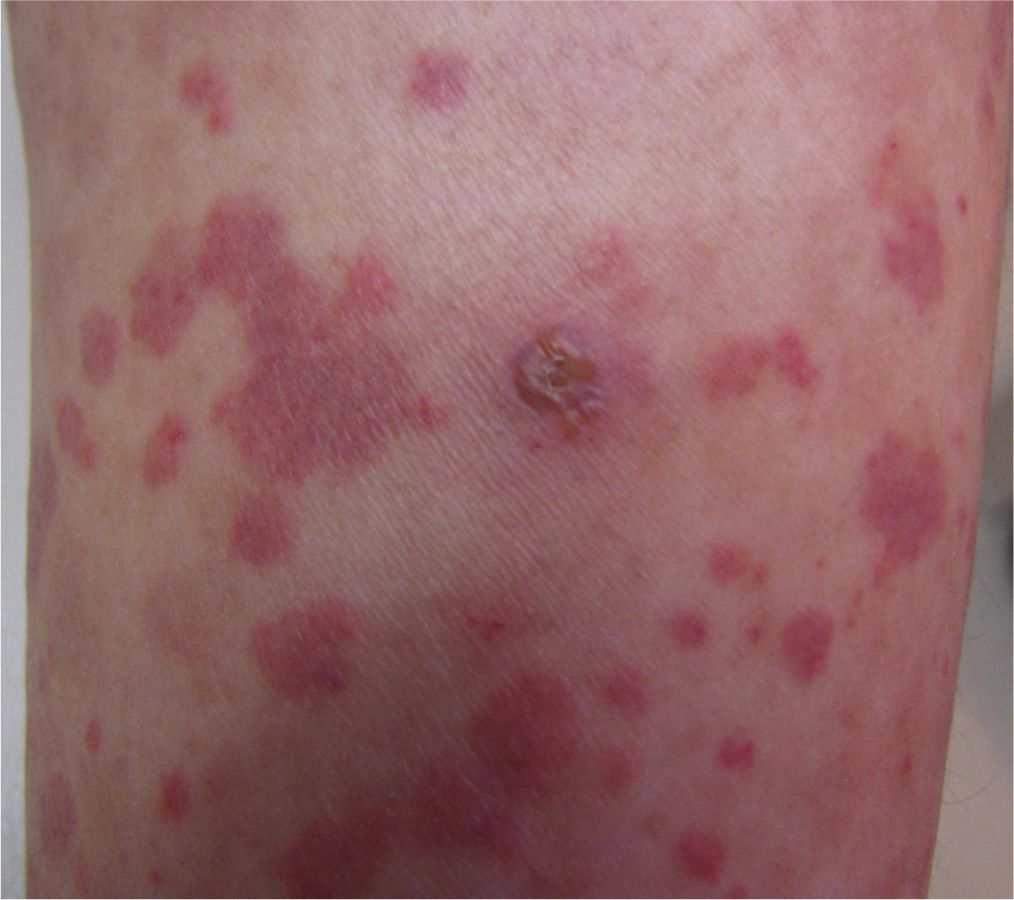
FIG. 16-5. Purpura with a bulla on the lower leg.
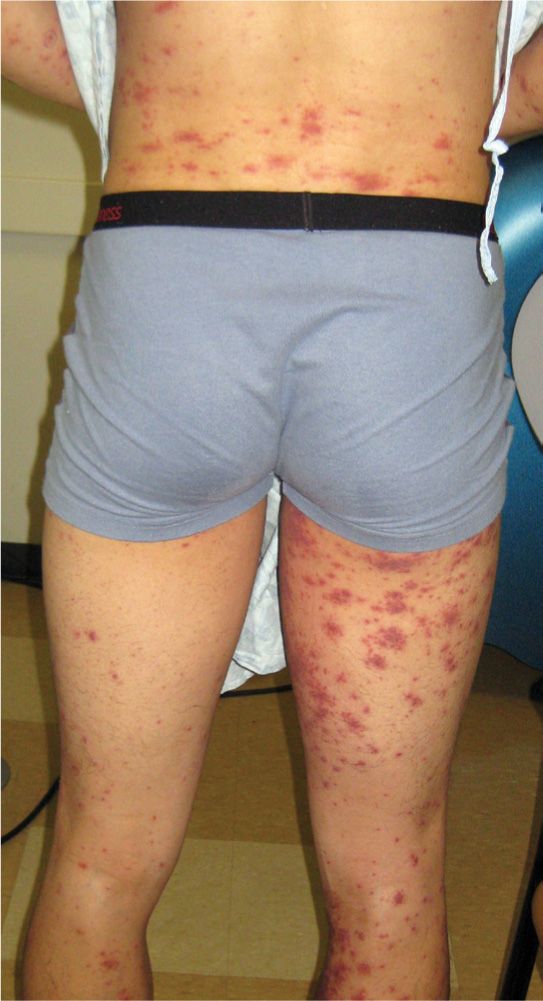
FIG. 16-6. Nonpalpable purpura of leukocytoclastic vasculitis, also called single-organ vasculitis, on man with an allergy to shellfish.
Assessment of the Patient with Cutaneous Vasculitis |
History | Timing, lesions develop 7–10 days after inciting agent. Special focus on history of malignancy, infections, connective tissue disorder, and recent drug administration or ingestion, including illicit drug use |
Review of systems | Critical for assessment of systemic involvement: cardiovascular, pulmonary, abdominal, and neurologic symptoms |
Physical examination | Detailed: For the initial evaluation, assess patient for signs of systemic involvement. Special attention should be paid to dependent areas, body areas affected by trauma or tight clothing Constitutional: fever, weight loss, chills, night sweats, and fatigue Weight loss, myalgia, arthralgia Positive findings: diplopia, headache, eye or ear pain, cough, shortness of breath, hemoptysis, nausea, vomiting, diarrhea, abdominal pain, blood in stool, weakness, neuropathy, myalgia, arthralgia, hair loss, sicca symptoms, sinus tenderness, oral or nasal inflammation, otitis media, keratitis, hepatosplenomegaly, and lymphadenopathy |
Referral and Consultation
The initial presentation and evaluation of vasculitis may begin in the primary care office, and patients with localized or early vasculitis can be managed symptomatically with removal of an offending antigen. Most patients are referred to a dermatology provider when there is extensive cutaneous involvement, threatened organ involvement, and relapse or disease progression despite treatment. A number of specialists should be consulted based on findings, including a nephrologist, pulmonologist, cardiologist, and otolaryngologist. Surgical consult may be necessary if necrotizing or ulcerative lesions are present.
Prognosis and Complications
If a drug or infection is the cause, there may only be one episode with spontaneous resolution; however, if SVV is associated with a systemic disease, such as hepatitis C infection or rheumatoid arthritis, treatment considerations will be different. Ultimately, the best indicator of prognosis in any vasculitis with systemic involvement depends on the size of the vessel and organ involved. Long-term implications include the possibility of multiple episodes with recurrent crops of vasculitic lesions appearing for months or years.
Patient Education and Follow-up
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






