Fig. 25.1
Macroscopic anatomy of the femur osseous flap and the branches of the femoral artery
Transplantation Study (N = 10)
Ten composite skin/bone isograft transplantations were performed between Lewis (LEW; RT1l) rats. Composite cutaneous groin and femoral osseous flap was harvested based on the femoral artery and vein. The viability of the skin and bone was evaluated clinically and histologically in isotransplants.
Surgical Technique
We used a standardized 4 × 5 cm template to design the groin cutaneous flap on the anterior abdominal wall and medial thigh region of the rat (Fig. 25.2a). The cutaneous flap was harvested based on the superficial epigastric artery and vein (Fig. 25.2b).
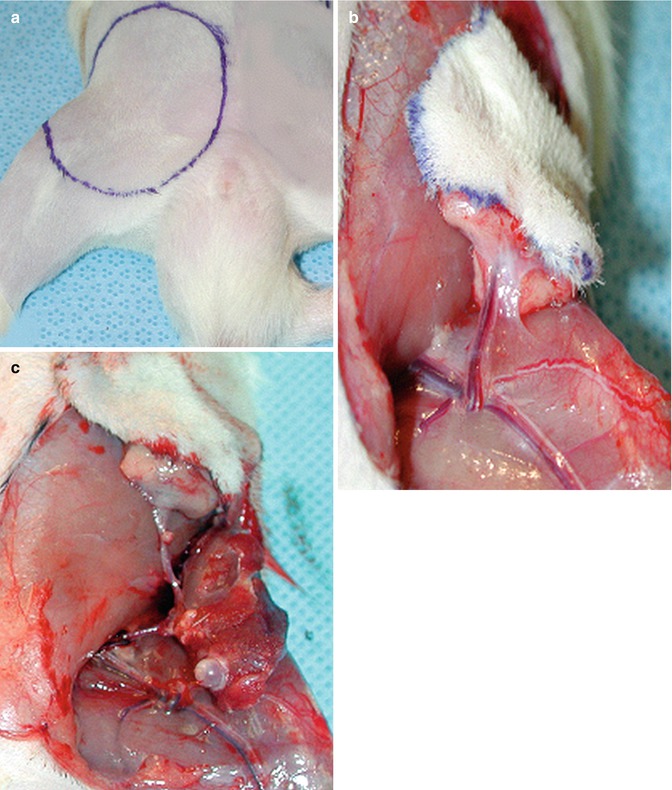

Fig. 25.2
Surgical procedure. (a) A standardized 4 × 5 cm template was used to design the groin cutaneous flap on the anterior abdominal wall and medial thigh region of the rat. (b) The cutaneous flap was harvested based on the superficial epigastric artery and vein. (c) Transplanted composite skin/bone flap. Note anastomosis between donor and recipient femoral artery and veins
Disarticulation of the Femoral Bone at the Level of the Knee Joint
The saphenous artery and vein were ligated and transected at the medial genicular region. Thereafter, at the medial thigh the gracilis and caudofemoralis muscles, at the posteromedial thigh the semitendinosus, semimembranosus and the medial head of the gastrocnemius muscles were divided from their insertions into the proximal tibia and distal femur. The posterior tibial nerves were transected and the popliteal artery and its tibial branches were ligated and transected. Superior genicular artery was identified and preserved at this level, The tendon of quadriceps femoris muscle (patellar ligament) was transected at the anterior thigh and the muscle was stripped proximally gently to protect the integrity of the periosteum. At the lateral side of the thigh tensor fascia lata muscle, which is attached to the lateral capsule was detached, as well as was the joint capsule. Biceps femoris muscle and the lateral head of the gastrocnemius muscle were divided posterolaterally, the cruciate ligaments within the joint were transected and finally the femur was disarticulated from the tibia.
Disarticulation of the Femoral Bone at the Level of the Hip Joint
The rat was placed in the lateral position on the table. First, gluteus maximus, tensor fascia lata and biceps femoris muscles were removed. Subsequently, abductor muscle group including quadratus femoris, internal and external obturator, and inferior and superior gemellus muscles were transected from their insertions. The femoral head was dissected out from the acetabulum after transection of the gluteus medius, gluteus minimus and piriformis muscles from their insertion at the greater trochanter. Two branches of the hypogastric artery, medial and lateral circumflex arteries, nourish the femoral neck and head. These arteries cannot be preserved since they arise from the hypogastric artery. Thus, femoral bone was disarticulated from the hip joint and the surrounding muscles were removed with the exception of rectus femoris muscle anteriorly, and adductor magnus, adductor longus, adductor brevis and pectineus medially. To be able to preserve the femoral bone survival, a small cuff of these muscles mentioned above should be preserved at their insertion points. Just on top of the rectus femoris and adductor magnus muscles there is femoral artery and its muscular branch. During the transection of the femoral vessels at the level of the inguinal ligament these muscles can be safely separated from their origin. Thus, all of the nourishing arteries of the femur could be preserved during the femoral bone flap isolation.
Transplantation of the Composite Vascularized Skin/Bone (VSB) Flap
Composite flap was flushed with cold heparinized saline (50 U in 10 ml) after transaction of the femoral vessels. A 4 × 5 cm defect was created in the anterior abdominal and medial thigh region of the recipient to accommodate the donor skin flap. The bone flap was placed in the inguinal region parallel and inferior to the inguinal ligament in an oblique position. In order to minimize the vascular pedicle tension, femoral head was positioned inferolaterally (Fig. 25.2c). Since the restricted inguinal space prevents excessive movement and twisting of the pedicle no tacking suture was required. End to end anastomosis of the femoral vessels was performed using standard microsurgical technique and 10/0 nylon. Skin was sutured in an interrupted fashion with 4/0 absorbable suture (Vicryl®, Ethicon, Inc.).
Clinical Evaluation of the Flap Viability
All transplants were evaluated on a daily basis for any signs of vascular failure. In the early postoperative period skin flaps were observed for color and temperature changes, hematoma formation, progressive edema, and later for hair loss, desquamation, epidermolysis, exudation and stiffness.
Histological Evaluation
The viability of the composite skin/bone flap components were evaluated histologically. Skin biopsies were obtained from the transplanted flap and fixed in 10 % formalin solution. Then fixed skin samples were embedded in paraffin blocks and 3 μm sections were prepared and stained with hematoxylin-eosin. We graded histological rejection pattern of the skin as described by Buttemeyer et al. [40] and Lee et al. [41]: grade 0 normal epidermal appearance without evidence of rejection, grade 1: focal mononuclear cells infiltration, grade 2: suprabasal bulla formation grade 3: vasculitis and complete skin necrosis with dermo-epidermal junction separation.
Biopsies were taken from femoral bone as well. Femoral bone samples were fixed and decalcified in 5 % formic acid solution for 3 days. Thereafter the fixed specimens were embedded in paraffin blocks and 3 μm sections were prepared and stained with Periodic Acid Schiff (PAS) and Giemsa dyes. Histological sections obtained from femoral bone were evaluated by an experienced pathologist for the viability and productivity of the bone marrow.
Results
Anatomic Study Outcome
Anatomic dissection showed that the vascular supply of the groin flap was coming directly from the superficial epigastric artery. This artery takes off from the superficial aspect of the femoral artery. Femoral bone’s circulation was supplied by four arteries [42] (Fig. 25.1). The medial and lateral circumflex arteries supplies blood to the proximal part of femoral bone. These branches arise from the hypogastric artery, at a much higher level in the pelvis. The muscular branch enters the femoral bone shaft in the mid-thigh level, near the insertion of the adductor longus and brevis, and pectineus muscles, and supplies the shaft of the bone. Superior genicular artery, arises from the deep surface of the femoral artery, usually just above the knee. This arterial branch can be identified during the deep dissection of the femoral shaft, between vastus medialis and adductor muscles. Ultimately, femoral bone receives its blood supply from the muscular and the superior genicular branches of the femoral artery.
Isograft Transplantation Study
Mean time for the composite skin/bone graft harvesting ranged from 10 to 20 min. Isograft transplantation time was around 45 min, which mean that total ischemia time was about 45 min. Flap transplantation was performed successfully in all ten animals. All rats tolerated the operation well and they returned to their normal activities at the first post-transplantation day. Body weight was stable and no infection signs were noticed. Mild to moderate hematoma formation was observed under the skin flap in four animals, which is resolved spontaneously within 8–12 days. Clinically, all flaps were healthy and pliable during the entire observation period. New hair growth was observed within 20–25 days post-transplant. No auto-cannibalization was observed.
Histological Evaluation
Normal (grade 0) skin histology and a viable compact and cancellous bone in the entire femur except the femoral head was observed in histological evaluation. There was an evident active hematopoiesis of the transplanted bone marrow (Fig. 25.3a, b).
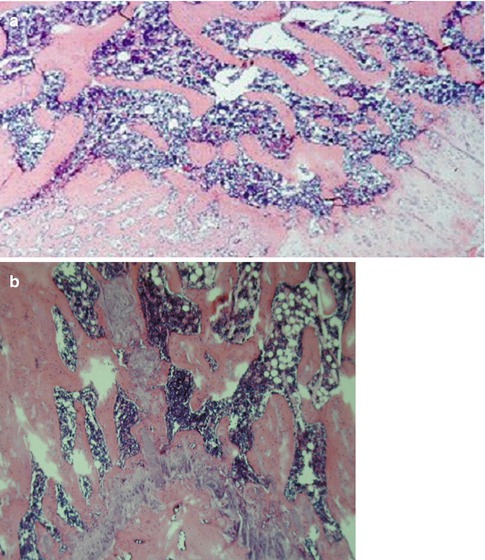

Fig. 25.3
Histological evaluation. (a) Histologic view of the metaphysis, and epiphysial plate of the greater trochanter showing viable compact and cancellous bone, bone marrow with active hematopoiesis, epiphysial plate with active endochondral ossification, and active reactive subperiosteal, radial bone growth (intramembranous ossification) (PAS, ×60). (b) Diaphysis of the femur with viable cortical bone and bone marrow, note active hematopoiesis (Giemsa, ×30)
Radiological Evaluation
Radiological evaluation using barium sulfate as a contrast agent revealed that the main arterial branches supplying the femoral bone and skin flap were well preserved within the flap.
Discussion
When we are talking about the transplantation of the composite tissues we are dealing with different types of tissue components with different antigenic properties including skin, tendons, nerves, muscle, bone etc. Currently there is a substantial demand for routine composite tissue allograft (CTA) transplantation; nevertheless, the necessity for life-long immunosuppression is an important obstacle for routine clinical applications.
Technical Aspects
Most of the information concerning the immunologic aspects of the vascularized bone marrow transplantation has been derived from the rat hind limb model, though it is a complex surgical procedure [43]. Moreover; the viability of the femoral bone marrow may be threatened during the limb amputation at the mid-femoral level and rigid fixation of the femur. Vascularized femur and vascularized sternum and are two other proposed models for bone marrow augmentation. The most important disadvantage of these techniques is the lack of a constant antigenic source such as skin, which is essential for CTA model. Furthermore, vascularized sternum transplantation is a quite complex procedure. New animal models are required to explain the details of molecular and cellular basis of tolerance induction in the experimental setting.
We eliminated most of these shortcomings accompanying with previous vascularized BM transplantation techniques in this new composite vascularized skin/bone transplantation model. In this model, vascularized femoral bone acts as a persistent and a consistent source for bone marrow delivery. We can expect a higher amount of bone marrow-derived cells engraftment when compared to the hind limb and vascularized sternum transplantation models because the femur contains a higher amount of bone marrow when compared with tibia and sternum. Moreover, femoral bone integrity is preserved in vascularized skin/bone transplantation model. In our vascularized skin/bone transplantation model, skin coming from the groin region was used as a component of these composite grafts and this unique feature allows for monitoring of graft viability in isograft setting, and rejection in allograft setting. Additionally, in vascularized skin/bone transplantation model the integrity of the recipient animal’s limb is preserved, making this experimental model more attractive for transplantation studies.
The microstructural integrity of the femur was preserved and histological analysis confirmed the viability of the bone and the skin. Femoral head was necrotic in histological bone marrow sections due to the sacrifice of medial and lateral circumflex femoral vessels during graft harvesting. Yet, more than 95 % of the bone marrow was expected to remain viable and could serve as a reliable source for donor cell engraftment. There was no complication observed that was associated with the necrosis of the femoral head.
Immunologic Aspects
Most of the immunologic knowledge regarding the skin allotransplantation is derived from non-vascularized skin allograft transplantation studies [1, 3–6]. An paramount factor for vascularized skin allograft transplantation is the migration of dendritic cells (DCs) through blood flow and lymph from the allograft to the host tissues. This is an important process that directly alters skin allograft rejection [7]. In a routine non-vascularized skin allograft transplant setting following the transplantation, it takes about 3–5 days for vascular in-growth from the recipient bed and 1 week for the restoration of lymphatic circulation [8




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








