8 Urodynamics: Voiding Studies
Normal micturition depends on a multitude of complex factors that must be coordinated to facilitate bladder emptying. Voiding consists of a combination of bladder contraction and outlet relaxation so that emptying is rapid and complete. The neurophysiologic mechanisms involved in micturition are complex and have been discussed elsewhere. Disturbances in any of the connections in the voiding mechanism can produce abnormal micturition.
UROFLOWMETRY
Uroflowmetry, or the measure of urine volume voided over time, is a simple and noninvasive test that is performed by asking the patient to void in a special commode. Urine is funneled into a flowmeter that records volume versus time (Fig. 8-1). A representative flow pattern is important to obtain, and the pattern depends on several factors. First, the patient should understand the simple nature of the test and be as relaxed as possible to have a normal desire to void at the time of the study. Second, the patient should be allowed to void in private because tension and embarrassment can artificially reduce the maximum flow achieved. Third, if there is doubt about the accuracy of the test, it is important to ask the patient whether he or she felt it was representative. If the patient believes that the test was not typical, it should be repeated.
Definitions and Normal Parameters
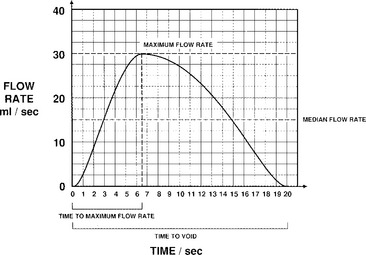
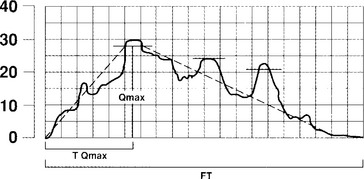
Urine flow may be described in terms of flow rate and flow pattern and may be continuous or intermittent. Flow rate (Q) is defined as the volume of fluid expelled via the urethra per unit time and is expressed in milliliters per second (mL/sec). Certain information is necessary in interpreting the tracing, including the volume voided, the environment and position in which the patient passed urine, whether the bladder filled naturally or by a catheter, and whether diuresis was stimulated by fluid or diuretics. If filling was by catheter, the type of fluid used should be stated; it should also be stated whether the flow study was part of another investigation. Maximum flow rate (Qmax) is the maximum measured value of the flow rate after correction for artifacts. Voided volume is the total volume expelled via the urethra. Flow time (Qtime) is the time over which measurable flow occurs. Average flow rate (Qave) is voided volume divided by flow time. The average flow should be interpreted with caution if flow is interrupted or if there is a terminal dribble. Time to maximum flow is the elapsed time from onset of flow to maximum flow. Drach et al. (1979) observed that the mean maximum flow rate in asymptomatic women was 26 ± 14 mL/sec, with an average voided volume of 224 mL. Flow time, maximum flow rate, and average flow rate all increase with corresponding increases in volume voided. Flow rates are higher in women than in men and in pregnant versus nonpregnant women. Little variation in flow rates with menstrual cycles, menopause, or increasing age has been reported.
Most experts agree that one can consider a study normal if the patient voids at least 200 mL over 15 to 20 seconds, and it is recorded as a smooth single curve with a maximum flow rate greater than 20 mL/sec (Fig. 8-2). Maximum flow rates of less than 15 mL/sec, with a voided volume greater than 200 mL, are generally considered abnormal. However, because flow rate is determined by the relationship between detrusor force and urethral resistance, and because these factors may vary considerably and still produce adequate bladder emptying, a precise definition of a normal or a low flow rate cannot be made. The maximum urinary flow rate (MUFR) and average urinary flow rate (AUFR) are the two most important uroflowmetry parameters.
Factors Affecting Flow Rates
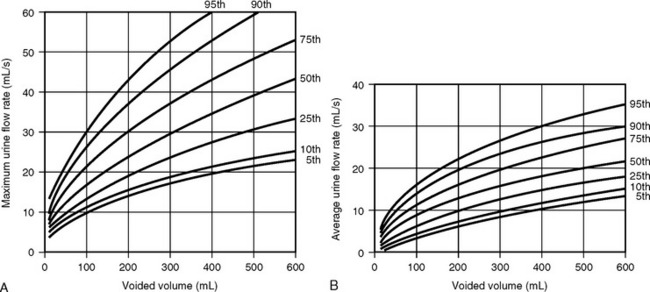
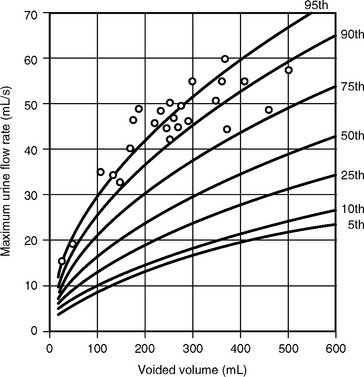
The clinical usefulness of uroflowmetry has been hampered by the lack of absolute values defining normal limits. These normal limits would need to be over a wide range of voided volumes ideally in the form of nomograms. Haylen et al. (1989) performed uroflowmetry on 249 female volunteers between ages 16 and 63. Uroflowmetry was performed on each woman in a completely private environment, and a second uroflow study was obtained in 46 of those women. The MUFR and the AUFR of the first voids were compared with respective voided volumes. By using statistical formulations of both voided volumes and urine flow rates, relationships between the two variables were obtained. This allowed the construction of nomograms that are shown in Figure 8-3. The study also showed that repetitive voiding does not seem to have an effect on flow rates (Fig. 8-4). Fantl et al. (1983) found no significant difference between the first and up to the sixth void in the 60 women who were tested. The use of nomograms overcomes the dangers of referencing flow rates to any one voided volume. A maximum flow rate of 15 mL/sec might fall just within the fifth centile curve at 200 mL voided volume, although well below the same curve at 400 mL. The previously described nomograms refer to free flowmetry voids and are not applicable to flow rates obtained during a pressure-flow study, because all urethral catheters can be expected to have the effect of decreasing urine flow rates for the equivalent voided volume. In 1999 Haylen et al. completed a further study of 250 women who were consecutive referrals for urogynecologic assessment, which included urodynamics secondary to symptoms of lower urinary tract dysfunction. Flow data for these women were converted to centiles from the Liverpool nomograms. They noted a decreased flow rate in symptomatic women, in general, and specifically in women with genital prolapse. Also, the data have shown that women who have undergone a hysterectomy have lower flow rates. In contrast to asymptomatic women, there is a decline in flow rates with age, and final urodynamic diagnosis shows that women with various combinations of stress incontinence, overactive bladder, or voiding dysfunction have flow rates that are significantly different than those of asymptomatic women.
Interpretation
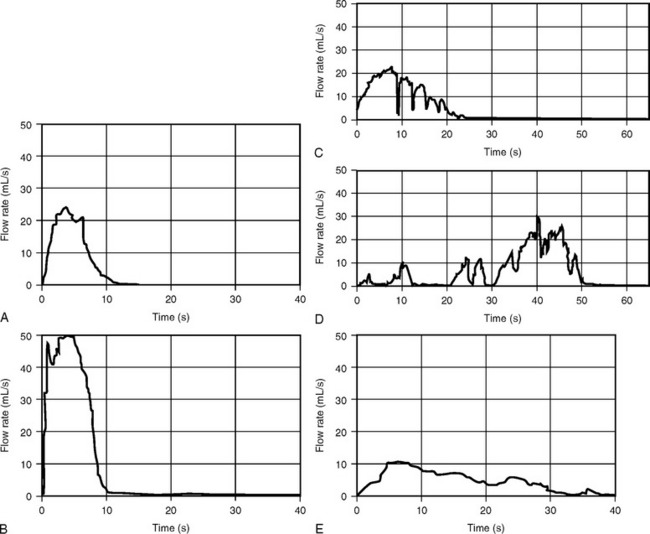
Curve patterns refer to the configuration of the uroflowmetric curve. Continuous flow that shows a rapidly increasing flow rate that reaches the maximum within one third of the total voiding time is usually considered normal (see Fig. 8-2; Fig. 8-5, A). Figure 8-5, B demonstrates what has been termed a superflow pattern in which there is a very rapid acceleration to a high MUFR.
Flow is considered intermittent when the flow rate drops and subsequently increases. Intermittent flow rates are described as multiple-peak patterns when there is a downward deflection of the flow rate that does not reach 2 mL/sec (Fig. 8-5, C). When the downward deflection of the flow rate reaches 2 mL/sec or less (Fig. 8-5, D), an interrupted pattern occurs. Uroflowmetric parameters can be obtained from multiple peak patterns by reconstructing the curve, as shown in Figure 8-6. The peak flow rate is determined by the highest horizontal segment that has a duration of at least 1 second. The peak flow rate is then connected to an ascending and descending limb. Deflections from the reconstructed curve are analyzed individually. Uroflowmetric parameters on curves with interrupted flow patterns are usually not estimated.
Obstructed voiding patterns are much less common in women than in men and usually produce a low, flat tracing (Fig. 8-5, E). Abnormal flow tracings caused by detrusor underactivity with abdominal straining (see Fig. 8-5, D) or by intermittent urethral sphincter activity (see Fig. 8-5, C) are characterized by slow changes in flow rate, producing a wavelike tracing. Each rise or fall in flow rate represents either a contraction of the abdominal and diaphragmatic muscles or a contraction of the external striated sphincter.
Detrusor contractility can be affected by neuropathic lesions, pharmacologic manipulation, intrinsic detrusor muscle or bladder wall dysfunction, or psychogenic inhibition.
Detrusor-external sphincter dyssynergia is a condition in which there is lack of coordination between the detrusor muscle and the external striated urethral sphincter. This leads to obstructed voiding and is always secondary to a neurologic lesion, most classically high spinal cord trauma. In the nervous and anxious but neurologically normal patient, the urethra may be closed by pelvic floor contracture. Urethral closure may be due to contraction of the intraurethral striated muscle or a contraction of the pelvic floor musculature. This condition has been termed detrusor sphincter pseudodyssynergia.