Introduction668
EPIDERMAL AND OTHER NEVI
EPIDERMAL NEVUS
Histopathology
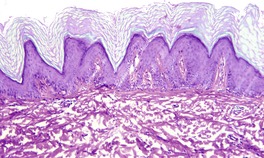
Fig. 31.1
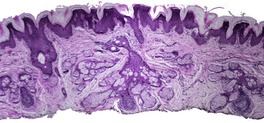
Fig. 31.2
INFLAMMATORY LINEAR VERRUCOUS EPIDERMAL NEVUS
Histopathology
NEVUS COMEDONICUS
Histopathology
FAMILIAL DYSKERATOTIC COMEDONES
Histopathology
PSEUDOEPITHELIOMATOUS HYPERPLASIA
GRANULOMA FISSURATUM
Histopathology
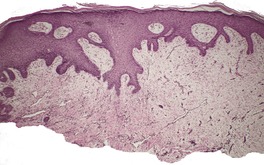
Fig. 31.3
PRURIGO NODULARIS
Histopathology165. and 181.
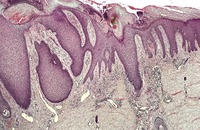
Fig. 31.4
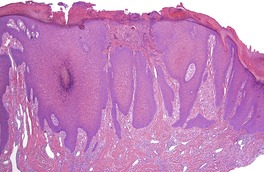
Fig. 31.5
ACANTHOMAS
EPIDERMOLYTIC ACANTHOMA
WARTY DYSKERATOMA
ACANTHOLYTIC ACANTHOMA
Histopathology198
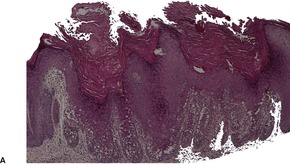
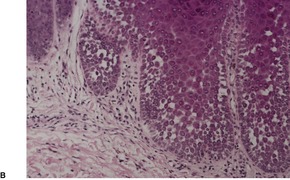
Fig. 31.6
SEBORRHEIC KERATOSIS
Histopathology197. and 235.
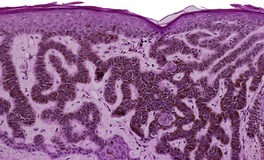
Fig. 31.7
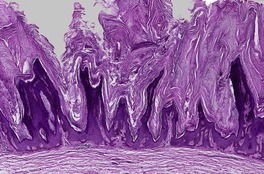
Fig. 31.8
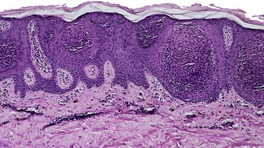
Fig. 31.9
Electron microscopy
Leser–Trélat sign
Histopathology287
DERMATOSIS PAPULOSA NIGRA
Histopathology313
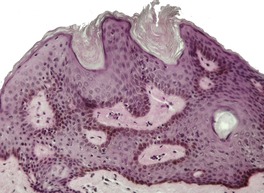
Fig. 31.10
MELANOACANTHOMA
Histopathology316.319. and 323.
CLEAR CELL ACANTHOMA
Histopathology328.332. and 350.
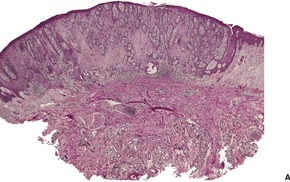
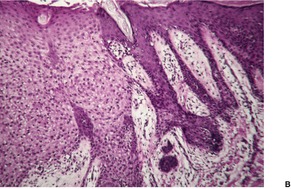
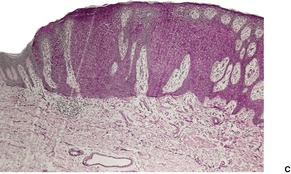
Fig. 31.11
CLEAR CELL PAPULOSIS
Histopathology
LARGE CELL ACANTHOMA
Histopathology369. and 370.
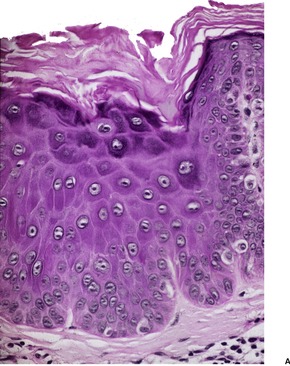
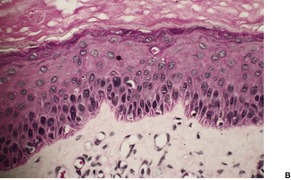
Fig. 31.12
EPIDERMAL DYSPLASIAS
ACTINIC KERATOSIS
Treatment of actinic keratosis
Histopathology467
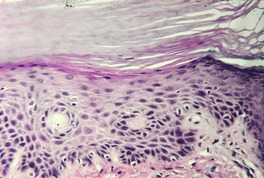
Fig. 31.13
Electron microscopy
ACTINIC CHEILITIS
Histopathology503. and 508.
ARSENICAL KERATOSES
Histopathology517. and 518.
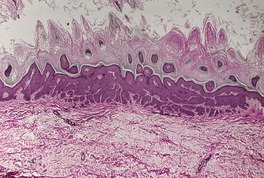
Fig. 31.14
PUVA KERATOSIS
Histopathology
INTRAEPIDERMAL CARCINOMAS
BOWEN’S DISEASE
Treatment of Bowen’s disease
Histopathology379. and 653.
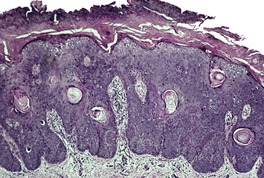
Fig. 31.15
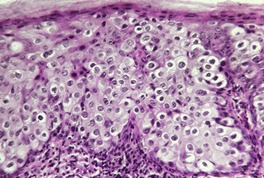
Fig. 31.16
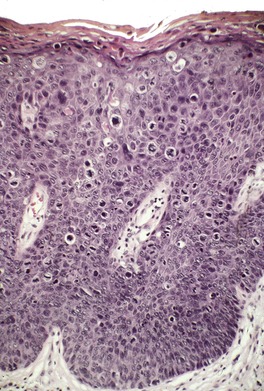
Fig. 31.17
Electron microscopy704
ERYTHROPLASIA OF QUEYRAT
INTRAEPIDERMAL EPITHELIOMA (JADASSOHN)
MALIGNANT TUMORS
BASAL CELL CARCINOMA
Nomenclature
Clinical aspects
Etiology
Genetic aspects
Cell of origin
Treatment of basal cell carcinoma
Histopathology1035
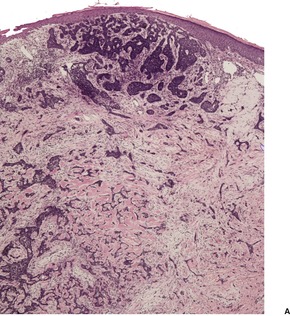
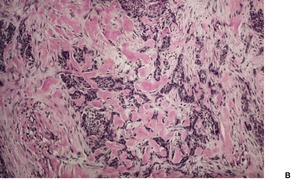
Fig. 31.18
Nodular (solid) type
Micronodular type
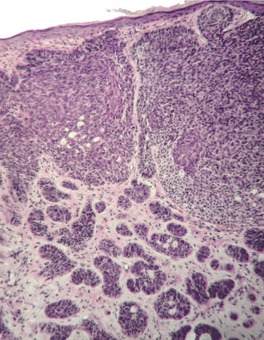
Fig. 31.19
Cystic type
Superficial (multifocal) type
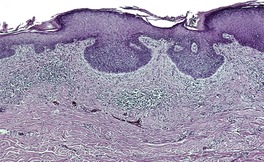
Fig. 31.20
Pigmented type
Adenoid type
Infiltrating type
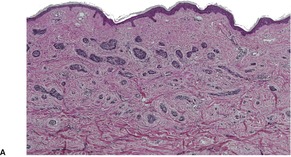
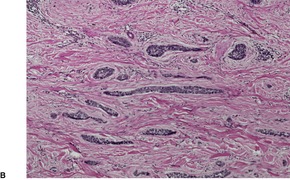
Fig. 31.21
Sclerosing type
Keratotic type
Infundibulocystic type
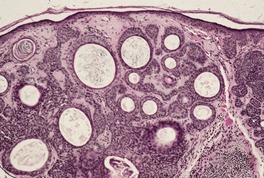
Fig. 31.22
Metatypical type
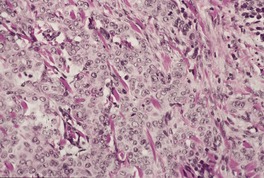
Fig. 31.23
Basosquamous carcinoma
Fibroepithelioma
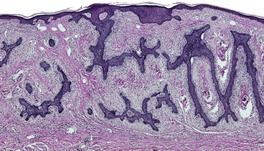
Fig. 31.24
Miscellaneous variants
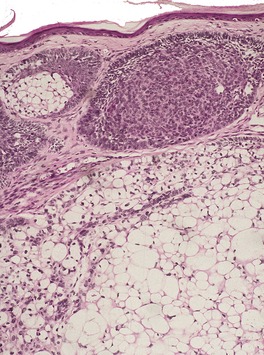
Fig. 31.25
Electron microscopy
Recurrences
Metastases
NEVOID BASAL CELL CARCINOMA SYNDROME
Histopathology1349
SQUAMOUS CELL CARCINOMA
Clinical aspects
Occurrence in organ transplant recipients
Etiology
Genetic aspects
Unitarian theory of squamous cell carcinoma
Treatment of squamous cell carcinoma
Histopathology
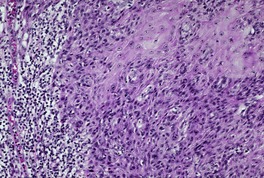
Fig. 31.26
Spindle-cell squamous carcinoma
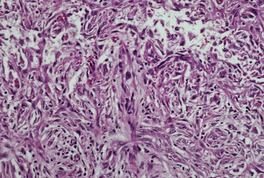
Fig. 31.27
Adenoid squamous cell carcinoma
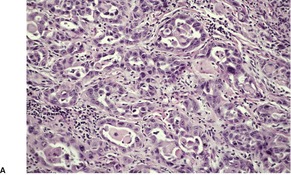
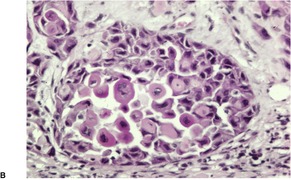
Fig. 31.28
Pseudovascular squamous cell carcinoma
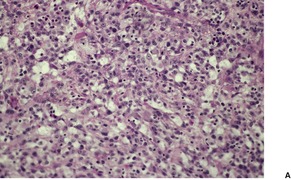
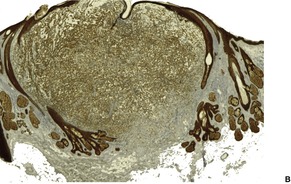
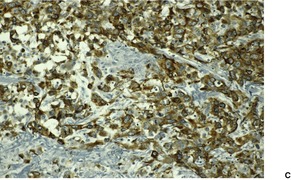
Fig. 31.29
Other variants of squamous cell carcinoma
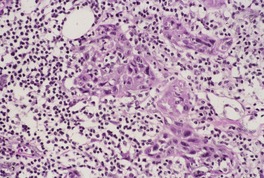
Fig. 31.30
Fig. 31.31
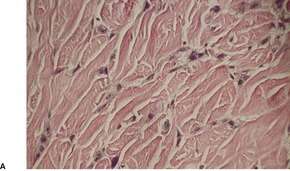
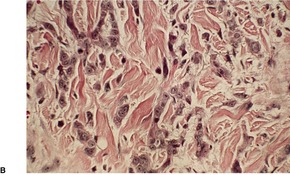
Fig. 31.32
Electron microscopy
Recurrences and metastases
VERRUCOUS CARCINOMA
Histopathology1735.1744.1748. and 1772.
Fig. 31.33
ADENOSQUAMOUS CARCINOMA
Histopathology1789. and 1790.
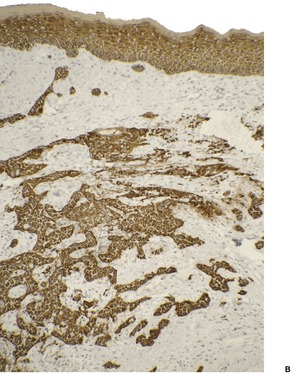
Fig. 31.34
MUCOEPIDERMOID CARCINOMA
Histopathology
CARCINOSARCOMA (METAPLASTIC CARCINOMA)
Histopathology
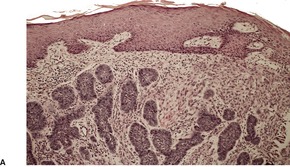
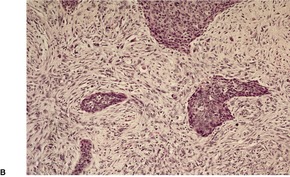
Fig. 31.35
LYMPHOEPITHELIOMA-LIKE CARCINOMA
Histopathology1829
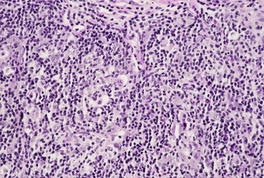
Fig. 31.36
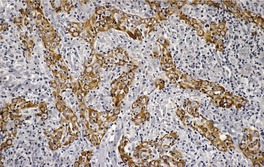
Fig. 31.37
MISCELLANEOUS ‘TUMORS’
CUTANEOUS HORN
Histopathology
STUCCO KERATOSIS
Histopathology1869.1870. and 1873.
CLAVUS (CORN)
Histopathology
Fig. 31.38
CALLUS
Histopathology
ONYCHOMATRIXOMA
Histopathology
KERATOACANTHOMA
Giant keratoacanthoma
Abortive keratoacanthoma
Keratoacanthoma centrifugum marginatum (multinodular keratoacanthoma)
Subungual keratoacanthoma
Multiple keratoacanthomas
Etiology
Treatment of keratoacanthoma
Histopathology
Fig. 31.39
Fig. 31.40
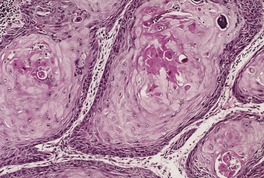
Fig. 31.41
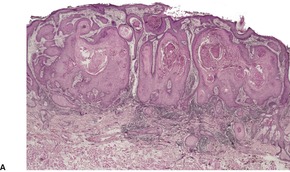
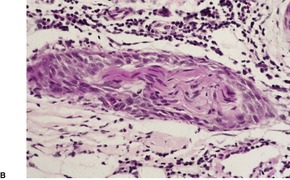
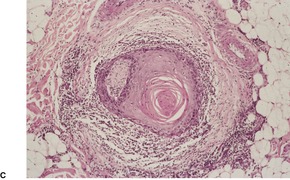
Fig. 31.42
Fig. 31.43
Fig. 31.44
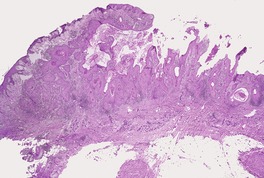
Fig. 31.45
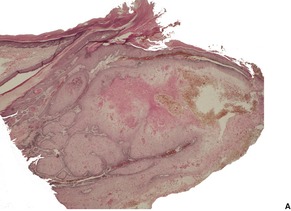
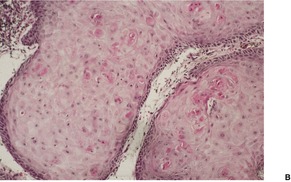
Fig. 31.46
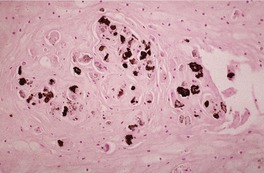
Fig. 31.47
Distinction of keratoacanthoma from squamous cell carcinoma
Behavior of keratoacanthomas
Metastasis
Regression
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tumors of the epidermis
Some authors use the term ‘epithelial nevus’ or ‘epidermal nevus’ as a group generic term to cover malformations of adnexal epithelium, as well as those involving the epidermis alone (see below).1. and 2. The term ‘epidermal nevus’ is used here in a restricted sense and does not include organoid, sebaceous, eccrine, and pilar nevi. These are considered with the appendageal tumors in Chapter 33. An exception has been made for nevus comedonicus, an abnormality of the infundibulum of the hair follicle. It is considered here because its histological appearance suggests an abnormality of the epidermis, rather than of appendages. Furthermore, the report of the coexistence of nevus comedonicus and an epidermal nevus suggests that the two entities are closely related. 3 The nevus comedonicus syndrome is regarded as a variant of the epidermal nevus syndrome; it has also been grouped with the organoid nevus (see p. 806).
The nomenclature of epidermal nevi has become confusing with some authors using the term as a group generic expression to cover all hamartomatous lesions derived from epidermal components, and resulting from mutations in early embryonic stages of development. They are then subclassified on the basis of their main constituent. The term ‘epidermal nevus’, as used here, is called keratinocyte epidermal nevus (OMIM 162900) in this new system. 4 Some cases have several components (keratinocyte, sebaceous, and eccrine), highlighting the difficulties created by this new nomenclature. 5
An epidermal nevus is a developmental malformation of the epidermis in which an excess of keratinocytes, sometimes showing abnormal maturation, results in a visible lesion with a variety of clinical and histological patterns. 6 They are hamartomatous lesions with an incidence of approximately 1 in 1000 live newborns. 7 Such lesions are of early onset, with a predilection for the neck, trunk, and extremities.8. and 9. There may be one only, or a few small warty brown or pale plaques may be present. A facial variant that was bilateral and symmetric has been reported. 10 At other times the nevus takes the form of a linear or zosteriform lesion, or just a slightly scaly area of discoloration.6.11. and 12. The acanthosis nigricans form of epidermal nevus, which may be zosteriform or oriented along Blaschko’s lines, appears to be a mosaic form of acanthosis nigricans. 13 Various terms have been applied, not always in a consistent manner, to the different clinical patterns. 12 The term ‘nevus verrucosus’ (verrucous epidermal nevus) has been used for localized wart-like variants,14.15. and 16. and ‘nevus unius lateris’ for long, linear, usually unilateral lesions on the extremities. 17‘Ichthyosis hystrix’ refers to large, often disfiguring nevi with a bilateral distribution on the trunk.18. and 19. Mosaicism for chromosome 6 has been found in skin fibroblasts from skin attached to an epidermal nevus. 20 Much more common are mutations in fibroblast growth factor receptor 3 (FGFR3), an abnormality found also in acanthosis nigricans and some seborrheic keratoses. 21
Various tumors have been reported arising in epidermal nevi, as a rare complication. These include basal cell22 and squamous cell carcinomas,23.24. and 25. as well as a keratoacanthoma. 26 Acne developed at puberty in one epidermal nevus. 27 Verrucous epidermal nevus (nevus verrucosus) has been reported in association with an organoid nevus (nevus sebaceus). 28 Colocalization with psoriasis has also been reported. 29
The term ‘epidermal nevus syndrome’ (OMIM 163200) refers to the association of epidermal nevi with neurological, ocular, and skeletal abnormalities, such as epilepsy, learning disability, hypophosphatemic vitamin D-resistant rickets, 30 cardiac arrhythmia, 31 cataracts, kyphoscoliosis, and limb hypertrophy; there may also be cutaneous hemangiomas.2.7.32.33.34.35.36.37.38.39.40.41.42. and 43. There are potentially many different epidermal nevus syndromes based on variable genetic mosaicism.9.44. and 45. The nevus comedonicus syndrome, CHILD syndrome (see p. 256), Proteus syndrome (see below), Becker’s nevus syndrome (OMIM 604919), and Schimmelpenning syndrome (OMIM 163200) have all been regarded as variants of epidermal nevus syndrome. 46 Cases with more traditional epidermal nevi have been called keratinocytic epidermal nevi. 47 Systemic cancers of various types may arise at a young age in those with the syndrome.48. and 49. The epidermal nevi, which are often particularly extensive in patients with the syndrome, may be of any histological type.50.51. and 52. Epidermal nevi have also been reported in association with polyostotic fibrous dysplasia, 53 and with the Proteus syndrome (OMIM 176920), a very rare disorder with various mesodermal malformations.54.55.56.57.58. and 59. Other cutaneous manifestations of this disorder include lipomas, vascular malformations, cerebriform connective tissue nevi on the soles of the feet, and partial lipohypoplasia.60.61. and 62. It has been claimed that cases of Proteus syndrome with PTEN mutations63 are a separate entity and not Proteus syndrome. 59 Seven patients previously regarded as Proteus syndrome have been reclassified as a new syndrome. They featured congenital lipomatous overgrowth, vascular malformations and epidermal nevi (CLOVE syndrome). 64
Topical treatments such as retinoic acid or 5-fluorouracil have been used to remove the keratotic surface of epidermal nevi and improve the cosmetic appearance. Cryotherapy is often followed by recurrence. Small lesions can be removed by surgical excision. Laser therapy can be used for larger lesions. Scarring may follow the use of the CO2 laser, but the pulsed erbium:YAG laser seems to give excellent cosmetic results. 65
At least 10 different histological patterns have been found in epidermal nevi (Fig. 31.1 and Fig. 31.2).6. and 12. More than one such pattern may be present in a given example. In over 60% of cases the pattern is that of hyperkeratosis, with papillomatosis of relatively flat and broad type, together with acanthosis. There is thickening of the granular layer and often a slight increase in basal melanin pigment. This is the so-called ‘common type’ of epidermal nevus.
Epidermal nevus. There is papillomatosis and acanthosis with overlying laminated hyperkeratosis. (H & E)
Epidermal nevus. This variant resembles acanthosis nigricans. (H & E)
Less frequently the histological pattern resembles acrokeratosis verruciformis, epidermolytic hyperkeratosis (OMIM 600648) or seborrheic keratosis.6. and 12. Epidermal nevi with a seborrheic keratosis-like pattern often have thin, elongated rete ridges with ‘flat bottoms’, a feature not usually seen in seborrheic keratoses. Rare patterns include the verrucoid, porokeratotic, focal acantholytic dyskeratotic,66.67.68.69. and 70. acanthosis nigricans-like,13. and 71. Hailey–Hailey disease-like, 72 and the incontinentia pigmenti-like (verrucous phase) variants. 73 A lichenoid inflammatory infiltrate has also been reported in an epidermal nevus, although usually the dermis is devoid of inflammatory cells. 74 Inflammatory linear verrucous epidermal nevus and nevus comedonicus are regarded as distinct entities, although they are sometimes included as histological patterns of epidermal nevus.
The epidermis overlying an organoid nevus (see p. 806) frequently will show the histological picture of an epidermal nevus. In such cases this is usually of the common type.
Also known by the acronym of ILVEN, inflammatory linear verrucous epidermal nevus is a specific clinicopathological subgroup of epidermal nevi which most often presents as a pruritic linear eruption on the lower extremities.2.75.76.77.78. and 79. The lesions are usually arranged along the lines of Blaschko. 80 The condition is of early onset, usually in the first 6 months of life. 81 Familial occurrence is rare. 82 Asymptomatic variants and widespread bilateral distribution have been reported;83. and 84. most cases are unilateral. ILVEN has been described in association with the epidermal nevus syndrome (see above), 85 and in a burn scar. 86
The lesions resemble linear psoriasis both clinically and histologically; in this context it must be noted that the existence of a linear form of psoriasis has been questioned.87. and 88. Interestingly, the epidermal fibrous protein isolated from the scale in ILVEN is different from that found in psoriasis.89. and 90. Because of the similarities between these two conditions etanercept has been used to treat widespread ILVEN. 91
The most widely used treatment is intralesional or potent topical corticosteroids. Both provide temporary relief at best. 81 Topical calcipotriol may be of some benefit, but it should be used with caution in children. 81 Destructive therapies have also been disappointing. Small lesions can be surgically removed.
There is psoriasiform epidermal hyperplasia with overlying areas of parakeratosis, alternating with orthokeratosis. Beneath the orthokeratotic areas of hyperkeratosis there is hypergranulosis, often with a depressed cup-like appearance; the parakeratosis overlies areas of agranulosis of the upper epidermis. 92 The zones of parakeratosis are usually much broader than in psoriasis. Focal mild spongiosis with some exocytosis and even vesiculation may be seen in some lesions. 93 There is also a mild perivascular lymphocytic infiltrate in the upper dermis. A dense lichenoid infiltrate was present in one case, perhaps representing the attempted immunological regression of the lesion. 94
A case reported as ILVEN of the penis and scrotum, and associated with ipsilateral undescended testicle is probably a variant of epidermal nevus with epidermolytic hyperkeratosis, based on the published photomicrographs. 95
The immunohistochemical features of ILVEN differ from those found in epidermal nevi. 96 This may simply be a reflection of the inflammatory component of ILVEN.
Nevus comedonicus (comedo nevus) is a rare abnormality of the infundibulum of the hair follicle in which grouped or linear comedonal papules develop at any time from birth to middle age.97.98. and 99. Approximately 50% of cases are evident at birth. 100 They are usually restricted to one side of the body, particularly the face, trunk, and neck.101.102. and 103. Rare clinical presentations have included penile, 104 scalp, 105 palmar,106.107. and 108. bilateral, 109 and verrucous lesions. 106 Extensive lesions are an uncommon manifestation. 110 Rarely, abnormalities of other systems such as the skeletal and neurological systems are present, indicating that a nevus comedonicus syndrome, akin to the epidermal nevus syndrome, occurs.81.111.112.113.114. and 115. It is regarded as a subset of the epidermal nevus syndrome. 47 Accessory breast tissue was present in one patient. 116 Nevus comedonicus has been reported in association with hidradenoma papilliferum and syringocystadenoma papilliferum. 117 All lesions were in the female genital area. 117 Inflammation of the lesions is an important complication, resulting in scarring.109.118.119. and 120.
Topical ammonium lactate, urea, and retinoids have all been tried at some time to treat nevus comedonicus. 115 Antibiotics are required for infected lesions. Small lesions can be excised. For large lesions the treatment of choice remains to be determined. 115 The erbium:YAG laser is a possibility for these larger lesions. 121
There are dilated keratin-filled invaginations of the epidermis. An atrophic sebaceous or pilar structure sometimes opens into the lower pole of the invagination. 122 A small lanugo hair is occasionally present in the keratinous material. Filaggrin expression is increased in the closed comedones seen in this condition. 123 Inflammation and subsequent dermal scarring are a feature in some cases. A tricholemmal cyst has also been reported arising in a comedo nevus. 124
The epithelial invagination in some cases of palmar involvement has opened into a recognizable eccrine duct.107. and 125. Cornoid lamellae have also been present.126.127. and 128. It has been suggested that these are cases of eccrine hamartomas, akin to nevus comedonicus and unrelated to porokeratosis. 129
Rare histological patterns associated with comedo nevus-like lesions have included a basal cell nevus, 130 a linear variant with underlying tumors of sweat gland origin, 131 and a variant with epidermolytic hyperkeratosis in the wall of the invaginations. 132 Epithelial proliferation, resembling that seen in the dilated pore of Winer, has been noted. 133 A form with dyskeratosis, accompanied often by acantholysis in the wall, is regarded as a distinct entity – familial dyskeratotic comedones (see below).
Familial dyskeratotic comedones is a rare autosomal dominant condition in which multiple comedones develop in childhood or adolescence, sometimes in association with acne.134.135.136. and 137. Sites of involvement include the trunk and extremities and, uncommonly, the palms and soles, the scrotum and the penis.134. and 138. This entity appears to be distinct from nevus comedonicus, and also from the rare condition of familial comedones.139. and 140.
A follicle-like invagination in the epidermis is filled with laminated keratinous material. Dyskeratotic cells are present in the wall of the invagination, particularly in the base. This is associated with acantholysis, which may, however, be mild or inapparent. 135
Pseudoepitheliomatous (pseudocarcinomatous) hyperplasia is a histopathological reaction pattern rather than a disease sui generis.141.142. and 143. It is characterized by irregular hyperplasia of the epidermis which also involves follicular infundibula and acrosyringia.141. and 144. This proliferation occurs in response to a wide range of stimuli comprising chronic irritation, including around urostomy and colostomy sites, 145 trauma, the use of permanent make-up, 146 a tattoo site, 147 cryotherapy, chronic lymphedema, and various dermal inflammatory processes such as chromomycosis, sporotrichosis, aspergillosis, pyodermas, bacillary angiomatosis, and actinomycosis.142.143.148. and 149. The lesions occurring around urostomy and colostomy sites take the form of pseudoverrucous papules and nodules. 150 Epidermodysplasia verruciformis (EV)-related human papillomaviruses have been reported in these lesions, but specifically excluded in other cases. 151 Pseudoepitheliomatous hyperplasia has been reported in the chronic verrucous lesions that develop in immunocompromised patients who develop herpes zoster/varicella infection (see p. 618). It may also develop in the halogenodermas and in association with chondrodermatitis nodularis helicis, 152 Spitz nevi, malignant melanomas, overlying granular cell tumors, cutaneous T-cell lymphomas, 153 and CD30+ lymphoproliferative disorders. 154 The term ‘pseudorecidivism’ has been used for the pseudoepitheliomatous hyperplasia that sometimes develops a few weeks after treatment, by various methods, 155 of a cutaneous tumor.
On microscopic examination there are prominent, somewhat bulbous, acanthotic downgrowths which in many instances represent expanded follicular infundibula. 141 Ackerman stresses that these structures are epidermal in origin and not follicular. 156 The hyperplasia is not as regular as that seen in psoriasiform hyperplasia. The cells have abundant cytoplasm, which is sometimes pale staining. Unlike squamous cell carcinoma there are few mitoses and only minimal cytological atypia. Aneuploidy is present in a small number of cases. 157 Where the process overlies dermal inflammation, transepidermal elimination of the inflammatory debris may occur, resulting in intraepidermal microabscesses.
In addition to those conditions already mentioned, pseudoepitheliomatous hyperplasia occurs in granuloma fissuratum and prurigo nodularis: these conditions are discussed below.
Granuloma fissuratum158 (acanthoma fissuratum, 159 spectacle-frame acanthoma160) is a firm, flesh-colored or pink nodule with a grooved central depression which develops at the site of focal pressure and friction from poorly fitting prostheses such as spectacles.159. and 161. Accordingly, such lesions are found on the lateral aspect of the bridge of the nose near the inner canthus, 162 in the retroauricular region, 158 and, rarely, on the cheeks.
A similar lesion develops in the mouth as a result of poorly fitting dentures. The lesions are painful or tender and they may ulcerate. Clinically they resemble basal cell carcinomas, although the central groove corresponding to the point of contact with the spectacle frame usually allows a correct diagnosis to be made. 163 Lesions heal within weeks of correction of the ill-fitting prosthesis.
The lesion is characterized by marked acanthosis of the epidermis with broad and elongated rete pegs (Fig. 31.3). There is a central depression, corresponding with the groove noted macroscopically, and here the epidermis is attenuated and sometimes ulcerated.158. and 160. There is mild hyperkeratosis and often a prominent granular layer overlying the acanthotic epidermis; there may be parakeratosis and mild spongiosis in the region of the groove or adjacent to it.
Granuloma fissuratum. There is focal pseudoepitheliomatous hyperplasia. (H & E)
The dermis shows telangiectasia of the small blood vessels with an accompanying chronic inflammatory cell infiltrate, which is usually mild and patchy. The infiltrate includes plasma cells, lymphocytes, and some histiocytes. There is focal fibroblastic activity and focal dermal fibrosis, with mild hyalinization of collagen beneath the groove. There may be mild edema of the superficial dermis.
Prurigo nodularis is an uncommon disorder in which there are usually numerous persistent, intensely pruritic nodules. There is a female predominance. In a recent series of 72 cases, the ages ranged from 15 to 92 years, with a median age at presentation of 55 years. 164 They involve predominantly the extensor aspects of the limbs, often symmetrically, but they may also develop on the trunk, face, scalp, and neck.165.166.167. and 168. The nodules are firm, pink, and sometimes verrucous or focally excoriated. They are approximately 5–12 mm in diameter and range from few in number to over 100. 165 Solitary lesions also occur. The intervening skin may be normal or xerotic; sometimes there is a lichenified eczema. 165 There may be an underlying cause for the pruritus, such as a metabolic disorder, 164 bites, folliculitis, the pruritic eruption of HIV infection,169. and 170. or atopic state.165. and 171. It has arisen in a healed herpes zoster scar, an isotopic response. 172 Mycobacteria have been cultured from, or demonstrated in, tissue sections in nearly one-quarter of cases in one study. 173 The significance of this finding is uncertain; it has not been confirmed. The tretinoin derivative etretinate, used in the treatment of a range of skin conditions, has been incriminated in several cases. 174 Stress is sometimes a factor, although psychological factors may have been overstressed in the past. 175 Prurigo nodularis is regarded by some authors as an exaggerated form of lichen simplex chronicus (see p. 86). Substance P-immunoreactive nerves, and calcitonin gene-related peptide are markedly increased in lesions of prurigo nodularis. 176
Capsaicin, an alkaloid which interferes with the perception of pruritus and pain by depletion of neuropeptides in small sensory nerves in the skin, has been used successfully in the treatment of prurigo nodularis. 177 Thalidomide has also been used, but care should be taken to exclude an underlying peripheral neuropathy before commencing this drug.178.179. and 180. Topical and intralesional steroids, emollients, topical antipruritics, doxepin, and cryotherapy are other treatments that have been used.164. and 176.
There is prominent hyperkeratosis, often focal parakeratosis, and marked irregular acanthosis that often is of pseudoepitheliomatous proportions (Fig. 31.4). Sometimes the hyperplasia is more regular and vaguely psoriasiform in type. There may be pinpoint ulceration from excoriation. Mitoses are usually increased among the keratinocytes. A hair follicle is often present in the center of each acanthotic downgrowth (Fig. 31.5). 182
Prurigo nodularis. There is marked pseudoepitheliomatous hyperplasia. (H & E)
Prurigo nodularis. There is focal excoriation and bulbous rete pegs. (H & E)
The upper dermis shows an increase in small blood vessels, and there is often an increase in the numbers of dermal fibroblasts, some of which may be stellate. There are usually fine collagen bundles in a vertical orientation in the papillary dermis. The arrectores pilorum muscles may be prominent. A syringomatous proliferation of eccrine sweat ducts is a rare finding. 183 The inflammatory cell infiltrate in the dermis is usually only mild and includes lymphocytes, mast cells, histiocytes, and sometimes eosinophils. Extracellular deposition of eosinophil granule protein is usually present. 184 Epidermal mast cells and Merkel cells may be seen.181. and 185. The mast cells are increased in size and become more dendritic. 186 They are seen in close vicinity to nerves which functionally express increased amounts of nerve growth factor receptor (NGFr). 186
Hypertrophy and proliferation of dermal nerve fibers has been emphasized by most,187.188.189. and 190. but it was not a prominent feature in two large series of cases.181. and 191. Small neuroid nodules with numerous Schwann cells have been reported in the dermis.192. and 193. Immunohistochemistry has revealed that calcitonin gene-related peptide (CGRP) is expressed in increased amounts in nerve fibers in prurigo nodularis; this may be of significance in the recruitment of eosinophils and mast cells into lesional tissue. 194 The CD10 preparation shows an onion skin-like cuff of positively staining dermal cells surrounding the hyperplastic downgrowths of epidermis. 195
Acanthomas are benign tumors of epidermal keratinocytes. 197 The proliferating cells may show normal epidermoid keratinization or a wide range of aberrant keratinization, including epidermolytic hyperkeratosis (epidermolytic acanthoma), dyskeratosis with acantholysis (warty dyskeratoma), or acantholysis alone (acantholytic acanthoma). 197 These abnormal forms of keratinization occur in a much broader context and have already been discussed in Chapter 9 (pp. 264–267). Brief mention of these forms of acanthoma is made again for completeness. Keratoacanthomas (see p. 702) are not usually considered in this category, although there is no logical reason for their exclusion. The following acanthomas are discussed below:
• epidermolytic acanthoma
• warty dyskeratoma
• acantholytic acanthoma
• seborrheic keratosis
• dermatosis papulosa nigra
• melanoacanthoma
• clear cell acanthoma
• clear cell papulosis
• large cell acanthoma.
Epidermolytic acanthoma is an uncommon lesion which may be solitary, resembling a wart, or multiple. It shows the histopathological changes of epidermolytic hyperkeratosis and is therefore considered in Chapter 9 with other lesions showing this disorder of epidermal maturation and keratinization (see p. 265).
Warty dyskeratomas are rare, usually solitary, papulonodular lesions with a predilection for the head and neck of middle-aged and elderly individuals (see p. 271). They show suprabasilar clefting, with numerous acantholytic and dyskeratotic cells within the cleft and an overlying keratinous plug.
Acantholytic acanthoma is a solitary tumor with a predilection for the trunk of older individuals.198. and 199. It usually presents as an asymptomatic keratotic papule or nodule. Uncommonly it may resemble a molluscum contagiosum clinically. 200 Multiple lesions have been reported in a renal transplant recipient. 201
The features include variable hyperkeratosis, papillomatosis and acanthosis, together with prominent acantholysis, most often involving multiple levels of the epidermis (Fig. 31.6). There is sometimes suprabasilar or subcorneal cleft formation, but there is no dyskeratosis. The pattern resembles that seen in pemphigus or Hailey–Hailey disease, but there has been no evidence of these diseases in the cases reported.
(A), (B) Acantholytic acanthoma. Acantholysis involves the lower layers of the hyperplastic epidermis. There is a thick, orthokeratotic stratum corneum. (H & E)
Seborrheic keratoses (senile warts, basal cell papillomas) are common, often multiple, benign tumors which usually first appear in middle life.202. and 203. They may occur on any part of the body except the palms and soles, although there is a predilection for the chest, interscapular region, waistline, and forehead. Seborrheic keratoses are sharply demarcated gray-brown to black lesions, which are slightly raised. They may be covered with greasy scales. Most lesions are no more than a centimeter or so in diameter, but larger variants, sometimes even pedunculated, have been reported.204.205. and 206. A flat plaque-like form is sometimes found on the buttocks or thighs. This should not be confused with the lightly pigmented plaques of unwashed skin which have variously been called keratoderma simplex, 207 dermatitis artefacta, and ‘terra firma-forme’ dermatosis (see p. 299). Rarely it arises in a nevus sebaceus. 208
Rare clinical variants include a familial form, which may be of early or late onset,209.210. and 211. and a halo variant with a depigmented halo around each lesion. 212 The Meyerson phenomenon (eczematous halo) may rarely involve a seborrheic keratosis. 213 Multiple seborrheic keratoses may sometimes assume a patterned arrangement along lines of cleavage214 or a linear (‘raindrop’) pattern. 215 A keratin horn is sometimes present, particularly in the elderly. 216 The eruptive form associated with internal cancer (sign of Leser and Trélat) is discussed below. Inflammation has developed in seborrheic keratoses after docetaxel treatment. 217
The nature of seborrheic keratoses is still disputed. A follicular origin has been proposed. They have also been regarded as a late-onset nevoid disturbance, or the result of a local arrest of maturation of keratinocytes. 218 Of interest is the recent finding of somatic mutations in fibroblast growth factor 3 (FGFR3) in a subset of patients with adenoid and flat seborrheic keratoses.219. and 220. Increased age appears to be a risk factor for these mutations. Furthermore, their preferential occurrence in seborrheic keratoses of the head and neck suggests a causative role for cumulative lifetime ultraviolet light exposure. 220 Mutations in FGFR3 are also found in epidermal nevi and acanthosis nigricans. Somatic mutations in PIK3CA have also been reported. 211 Human papillomavirus (HPV) has been detected in a small number of cases, 221 particularly from the genital region. 222 It has also been found in the seborrheic keratoses of patients who have epidermodysplasia verruciformis.223. and 224. The DNA of epidermodysplasia verruciformis-associated human papillomaviruses has been detected in non-genital seborrheic keratoses. 225 Several cases of necrotizing herpesvirus infection complicating a seborrheic keratosis have been reported. 226 Endothelin-1, a keratinocyte-derived cytokine with a stimulatory effect on melanocytes, is thought to be involved in the melanization of seborrheic keratoses.227. and 228. The basosquamous cell acanthoma of Lund, the inverted follicular keratosis, and the stucco keratosis have all been regarded, at some time, as variants of seborrheic keratosis.197. and 229.
Dermoscopy is a reliable method of distinguishing these lesions from malignant melanoma and other pigmented lesions. The classic criteria – milia-like cysts and comedo-like openings – have a high prevalence but other features such as fissures, hairpin blood vessels, sharp demarcation, moth-eaten borders, and ‘fat fingers’ may also be present.230. and 231.
A case can be made for submitting all suspected seborrheic keratoses for histological examination as clinical misdiagnosis can occur.232. and 233.
Shave excision, curette and cautery, and cryotherapy have all been used to treat seborrheic keratoses. In Australia, excision is sometimes used but there is no Medicare reimbursement for such excisions. Tazarotene cream 0.1% was found to be cosmetically acceptable in a trial comparing cryosurgery, tazarotene, topical imiquimod, and topical calcipotriene. 234
Seborrheic keratoses are sharply defined tumors which may be endophytic or exophytic. 235 They are composed of basaloid cells with a varying admixture of squamoid cells. Keratin-filled invaginations and small cysts (horn cysts) are a characteristic feature. Nests of squamous cells (squamous eddies) may be present, particularly in the irritated type. The squamous eddies of irritated seborrheic keratoses are anatomically related to acrotrichia. 236 Approximately one-third of seborrheic keratoses appear hyperpigmented in hematoxylin and eosin-stained sections. 205
At least nine distinct histological patterns have been recognized: acanthotic (solid), reticulated (adenoid), hyperkeratotic (papillomatous), clonal, irritated, inflamed, desmoplastic, adamantinoid, and with pseudorosettes.235.237. and 238. Overlapping features are quite common. The acanthotic type is composed of broad columns or sheets of basaloid cells with intervening horn cysts. The reticulated (adenoid) type has interlacing thin strands of basaloid cells, often pigmented, enclosing small horn cysts (Fig. 31.7). This variant often evolves from a solar lentigo. 235 The hyperkeratotic type is exophytic, with varying degrees of hyperkeratosis, papillomatosis, and acanthosis (Fig. 31.8). There are both basaloid and squamous cells. Clonal seborrheic keratoses (Fig. 31.9) have intraepidermal nests of basaloid cells resembling the Borst–Jadassohn phenomenon (see p. 682). 239 In the irritated variant there is a heavy inflammatory cell infiltrate, with lichenoid features, in the upper dermis. Apoptotic cells are present in the base of the lesion and in areas of squamous differentiation. 240 This represents the attempted immunological regression of a seborrheic keratosis.241. and 242. The term lichenoid seborrheic keratosis is a preferable term to irritated seborrheic keratosis. Kamino bodies (see p. 721) are rarely found in this type. 243 Sometimes there is a heavy inflammatory cell infiltrate without lichenoid qualities; 244 rarely neutrophils are abundant in the infiltrate. 205 This may be regarded as a true inflammatory variant, although often lesions with features overlapping with those of the irritated (lichenoid) type are found. 244 In the desmoplastic variant there are irregular squamous nests and cords of cells extending into a desmoplastic stroma. The trapped cells may mimic a squamous cell carcinoma. 237 This variant is analogous to the desmoplastic tricholemmoma. A variant with intercellular mucin and small basaloid keratinoyctes with spindled cytoplasm has been called an adamantinoid seborrheic keratosis. 238 In the variant with pseudorosettes, basaloid cells are radially arranged around central, small empty spaces. The lesion is acanthotic with occasional horn cysts. 238
Reticulated (adenoid) seborrheic keratosis. There are irregular, acanthotic downgrowths. (H & E)
Seborrheic keratosis of hyperkeratotic type with marked papillomatosis and hyperkeratosis. (H & E)
Clonal seborrheic keratosis. Nests of keratinocytes show the Borst–Jadassohn phenomenon. (H & E)
Tricholemmal differentiation with glycogen-rich cells is an uncommon, usually focal, change.245. and 246. So too is sebaceous differentiation. 247 Acantholysis is another uncommon histological feature.248. and 249. Basal clear cells are sometimes present in seborrheic keratoses. These cells express keratin markers, often at the periphery of the cells, but not S100 or melan-A. They may mimic melanoma in situ on H & E sections. 250 Trichostasis spinulosa with multiple retained hair shafts has also been reported. 251 Amyloid in the underlying dermis is another incidental finding. 235 A verruciform xanthoma-like lesion has also developed in a seborrheic keratosis. 252
The development of a basal cell or squamous cell carcinoma or a keratoacanthoma in a seborrheic keratosis is a rare event.253.254.255.256.257.258.259.260. and 261. More common is the juxtaposition or ‘collision’ of these lesions.262. and 263. Another finding is epidermal atypia of varying severity in the cells of a seborrheic keratosis; a progressive transformation to in-situ squamous cell carcinoma (bowenoid transformation) may occur.264.265.266.267.268.269.270. and 271. Rarely, a malignant melanoma may develop in a seborrheic keratosis.260. and 272. In a recent study of 639 consecutive seborrheic keratoses, an associated (adjacent to or contiguous with) lesion was present in 85 cases (9%). 261
The sign of Leser and Trélat is defined as the sudden increase in the number and size of seborrheic keratoses associated with an internal cancer.276.277. and 278. Approximately 100 such cases have been reported,279.280.281. and 282. although some of them are poorly documented as genuine examples of the sign.283. and 284. Other cutaneous paraneoplastic conditions, such as acanthosis nigricans, hypertrichosis lanuginosa, and acquired ichthyosis, are sometimes present as well.285.286.287. and 288. Pruritus is also common. 279 A gastrointestinal tract adenocarcinoma is the most frequent accompanying cancer,289. and 290. followed by lymphoproliferative disorders.291.292.293. and 294. The various cancers reported with this sign are analyzed in several reviews and case reports.280.286.287. and 295. Metastases are frequently present and most patients have a poor prognosis.285. and 287.
The seborrheic keratoses may precede, 296 follow, or develop concurrently with the onset of symptoms of the cancer.287. and 297. Cases purporting to represent chemotherapy-induced lesions have been reported. 298 Involution of the seborrheic keratoses has followed treatment of the cancer. The sign has also occurred during the course of the disease. 299 The lesions are most frequent on the trunk. A variant in which they were linear in distribution has been reported. 300
The mechanism responsible for the appearance of these keratoses is not known. Epidermal growth factor does not appear to be increased, 290 although the structurally related α-transforming growth factor was increased in one report. 280 Elevated insulin-like growth factor was present in a patient with eruptive seborrheic keratoses and solitary fibrous tumor of the pleura. 301
Eruptive seborrheic keratoses have rarely been reported during the course of an erythrodermic condition.302. and 303. Transient, eruptive seborrheic keratoses have developed in patients with erythrodermic pityriasis rubra pilaris, 304 erythrodermic psoriasis, and an erythrodermic drug eruption. 305 They have also been reported in a patient with acromegaly, 306 in one with HIV infection, 307 and also in a heart transplant recipient. 308
Histological examination of the skin lesions has only been made in isolated cases. 309 In some reports typical seborrheic keratoses have been present. In other instances non-specific hyperkeratosis and papillomatosis without acanthosis have been noted. 287 Florid cutaneous papillomatosis with hyperkeratosis and acanthosis has also been described. 310 The regression of lesions following treatment of the underlying cancer appears to be associated with a mononuclear cell infiltrate in the upper dermis and lower epidermis. 303
Although regarded by some as a variant of seborrheic keratosis, 205 dermatosis papulosa nigra is a clinically distinctive entity, found almost exclusively in black adults, with a female preponderance. 311 A familial predisposition is often present. 312 There are multiple small pigmented papules, with a predilection for the malar area of the face. The neck and upper part of the trunk may also be involved. Lesions have been found in from 10% to 35% of the black population in the United States. 313 An eruptive form has been reported in association with an adenocarcinoma of the colon. 314
Electrodesiccation is the treatment of choice, particularly if multiple lesions are present. 312
Dermatosis papulosa nigra is characterized by hyperkeratosis, elongated and interconnected rete ridges, and hyperpigmentation of the basal layer (Fig. 31.10). There are often keratin-filled invaginations of the epidermis. The picture is similar to that of the reticulate type of seborrheic keratosis. In contrast to seborrheic keratoses, the epithelial proliferation in dermatosis papulosa nigra is not usually composed of basaloid cells.
Dermatosis papulosa nigra. There are interconnected rete pegs and hyperpigmentation of the basal layer. (H & E)
The term ‘melanoacanthoma’ was introduced in 1960 for a rare benign pigmented lesion which is composed of both melanocytes and keratinocytes. 315 The lesion is a slowly growing, usually solitary, tumor of the head and neck, or trunk, of older people.316.317. and 318. Clinically, a melanoacanthoma resembles a seborrheic keratosis or a melanoma, and may grow to 3 cm or more in diameter. 319 It is best regarded as a variant of seborrheic keratosis. Melanoacanthoma has been recorded as arising from mucous membranes: such lesions represent an unrelated disorder.320.321. and 322.
There is some resemblance to a seborrheic keratosis, with an acanthotic, slightly verrucous epidermis composed of both basaloid and spinous cells. 323 The basaloid cells sometimes form islands, whereas the spinous cells form foci with central keratinization and horn pearl formation (endokeratinization). Numerous dendritic melanocytes are scattered throughout the lesion. 316 The melanocytes contain mature melanosomes and are heavily pigmented. The neighboring keratinocytes are only sparsely pigmented. 324 There is usually pigment in macrophages in the upper dermis. The dendritic melanocytes express S100 protein and HMB-45. 322
Clear cell acanthoma (pale cell acanthoma) is an uncommon firm brown-red dome-shaped nodule or papule, 5–10 mm or more in diameter, with a predilection for the lower part of the legs of middle-aged and elderly individuals.325.326.327.328. and 329. Giant forms have been described.330. and 331. Rarely, other sites have been involved;332.333. and 334. onset in younger patients has also been recorded. 335 Although usually solitary, multiple tumors have been described and a few patients with multiple clear cell acanthomas have also had varicose veins and/or ichthyosis.336.337.338.339. and 340. In one case, the lesion developed over a melanocytic nevus. 341 Associated conditions, both adjacent to and underlying a clear cell acanthoma, are common. 342 The lesions may have a crusted surface and may bleed with minor trauma. A scaly collarette and vascular puncta on the surface of the lesion are common.325.343. and 344. Growth is slow and the tumor may persist for many years. Spontaneous involution has been reported. 345
The exact nosological position of this lesion is uncertain, but it has generally been considered to be a benign epidermal neoplasm (as originally proposed by Degos) rather than a reactive hyperplasia of inflammatory origin.332. and 346. It does not show tricholemmal differentiation. 342 However, the expression of cytokeratins is similar to that seen in some inflammatory dermatoses. 347 Furthermore, clear cell acanthoma has developed in a psoriatic plaque. 348
On dermoscopy, there is some resemblance to psoriasis, with a squamous surface and translucid collarette. 349 There are dilated capillary loops, with a mainly perpendicular orientation to the skin surface. 349
Clear cell acanthomas may be treated with excision, shave excision, cryotherapy, and curettage and electrofulguration. 334 Cryotherapy usually requires three to four courses. As many cases are misdiagnosed clinically as basal cell carcinomas, they are often surgically removed.
Histological examination shows a well-demarcated area of psoriasiform epidermal hyperplasia in which the keratinocytes have pale-staining cytoplasm. The epithelium of the adnexa is spared. There are intermittent broad and slender rete pegs, and a tendency for the acanthosis to be more prominent centrally. There may be fusion of the acanthotic downgrowths. Usually there is slight acanthosis of the epidermis, involving one or two rete ridges bordering the area of pale acanthosis (Fig. 31.11). 350
(A) Clear cell acanthoma. (B) The lesion is acanthotic, with pale-staining keratinocytes, except at the periphery of the lesion where they appear normal. (H & E) (C) The cells stain with the PAS stain.
Other epidermal changes include mild spongiosis, exocytosis of neutrophils which may form tiny intraepidermal microabscesses, and thinning of the suprapapillary plates. The epidermal surface shows parakeratotic scale and sometimes focal pustulation. The cytoplasm of the basal cells may not be as pale as that of the other keratinocytes: it is often devoid of melanin pigment, although melanocytes are present. 351 A pigmented variant with dendritic melanocytes has been reported. 352 Cellular atypia is a rare occurrence. 353
The dermal papillae are edematous, with increased vascularity and a mixed inflammatory cell infiltrate which includes a variable proportion of lymphocytes, plasma cells, and neutrophils. In several cases the sweat ducts have been dilated, and rarely they may be hyperplastic.
A PAS stain, with and without diastase, will confirm the presence of abundant glycogen in the pale cells. Electron microscopy has also confirmed that the keratinocytes contain glycogen.354. and 355. Langerhans cells are also abundant.338. and 356. Immunohistochemistry shows that the cells contain keratin and involucrin, but not carcinoembryonic antigen. 357
It has been suggested that there is a distinct tissue reaction, pale cell acanthosis (clear cell acanthosis), characterized by the presence of pale cells in an acanthotic epidermis. 358 This histological pattern can be seen not only in clear cell acanthoma but also in some seborrheic keratoses, usually the clonal subtype, and rarely in verruca vulgaris. The lesion reported as a cystic clear cell acanthoma may represent this tissue reaction occurring in an epidermal cyst or dilated follicle. 359
Clear cell papulosis is an exceedingly rare condition characterized by multiple white papules on the face, chest, abdomen, or lumbar region of young women and boys.360.361.362.363.364. and 365. There is a predisposition for persons of Asian or Hispanic descent. 366 Some lesions may develop along the ‘milk lines’. The lesions measure 2–10 mm in size. The number of lesions has ranged from 5 to more than 100. It has been suggested that there may be some histogenetic relationship with Toker’s clear cells of the nipple and that cases reported away from the ‘milk lines’ may be a different entity. 367
There is no known treatment. 365
The epidermis is mildly acanthotic with a slightly disorganized arrangement of the epidermal cells. The characteristic feature is the presence of clear cells scattered mainly among the basal cells, with a few cells in the malpighian layer. The cells are larger than the adjacent keratinocytes. The clear cells are variably stained by the PAS, mucicarmine, Alcian blue, and colloidal iron methods. A characteristic feature is the positive immunostaining for gross cystic disease fluid protein-15 (GCDFP). 360 They also stain positively for cytokeratin 7, CAM5.2, epithelial membrane antigen (EMA), and carcinoembryonic antigen (CEA), but they are negative for CD1a and S100 protein.366. and 368.
The clear cells in pagetoid dyskeratosis, an incidental histological finding in a variety of lesions, are found at a higher level in the epidermis (see p. 272). They do not stain with the PAS or mucicarmine methods.
Large cell acanthoma occurs as a sharply demarcated, scaly, often lightly pigmented patch, approximately 3–10 mm in diameter, on the sun-exposed skin of middle-aged and elderly individuals.369.370. and 371. It is usually solitary. Clinically, it resembles a seborrheic or actinic keratosis. Large cell acanthoma is thought to comprise sunlight-induced clones of abnormal cells, without a tendency to malignancy.369. and 372. However, the author has seen several cases with bowenoid transformation (see below). As such it is a distinctive condition373.374. and 375. and not a variant of solar lentigo, as proposed by Roewert and Ackerman, 376 and others. 377 Human papillomavirus type 6 (HPV-6) has been isolated from lesional skin in a patient with multiple large cell acanthomas. 378
There is epidermal thickening caused by the enlargement of keratinocytes to about twice their normal size (Fig. 31.12). There is also a proportional increase in nuclear size. The lesions are sharply demarcated from the adjacent normal keratinocytes; the adnexal epithelium within a lesion is usually spared. Other features include orthokeratosis, a prominent granular layer, mild papillomatosis, mild basal pigmentation, and some downward budding of the rete ridges. 375 Occasionally there is a focal lichenoid inflammatory cell infiltrate. Atypia may develop in large cell acanthoma; only rarely is this bowenoid.
Large cell acanthoma. (A) The keratinocytes are larger than usual and the granular layer is thickened. Normal epidermis is present at the edge of the photograph. (B) There is mild basal cell atypia. There would be parakeratosis overlying a solar keratosis. There is orthokeratosis here. (H & E)
The epidermal dysplasias have the potential for malignant transformation. This group includes actinic (solar) keratosis, actinic cheilitis, arsenical keratoses, and PUVA keratosis.
Actinic (solar) keratoses present clinically as circumscribed scaly erythematous lesions, usually less than 1 cm in diameter, on the sun-exposed skin of older individuals.379.380.381. and 382. The face, ears, scalp, hands, and forearms are sites of predilection. 383 In sunbed users, punctate dysplastic keratoses have developed, predominantly on the plantar aspect of the feet. 384 In Australia, actinic keratoses are found in 40–60% of people aged 40 years and over.385. and 386. They develop most often in those with a fair complexion, who do not tan readily. 387 They may also develop in lesions of vitiligo. 388 In contrast, in England the prevalence of actinic keratoses is approximately 15% for men and 6% for women. 389 The prevalence is also low in Italy and Japan. 390
Actinic keratoses may remit, or remain unchanged for many years.391.392.393. and 394. It has been stated that 8–20% gradually transform into squamous cell carcinoma if left untreated.379.395. and 396. The hyperplastic variant appears to have a relatively high rate of malignant transformation. 397 In one study the annual incidence rate of malignant transformation of a solar keratosis was less than 0.25% for each keratosis, 398 but this study has been criticized on several grounds.399. and 400. Patients with solar keratoses on the trunk or lower extremities are at high risk for skin cancer development. 401
Ackerman and others have proposed that actinic keratoses are morphological expressions of squamous cell carcinoma (see p. 693),402.403.404. and 405. while Cockerell has suggested that actinic (solar) keratoses be renamed ‘keratinocytic intraepidermal neoplasia’ or ‘solar keratotic intraepidermal SCC’.406. and 407. It is difficult to envisage clinicians embracing this latter terminology. This trend to abandon precursor diagnoses in favor of the worst scenario outcome has important implications for patient management and their peace of mind, not to mention its conflict with traditional views of the stepwise (multistage) progression of neoplasia.408.409.410.411.412. and 413. Recent work (2008) suggests that Bowen’s disease and actinic keratoses are derived from different cells. Basal cells appear to play a role in the histogenesis of actinic keratosis but not Bowen’s disease. 414
Several clinical variants of actinic keratosis have been described. In the hyperplastic (hypertrophic) form, found almost exclusively on the dorsum of the hands and the forearms, individual lesions are quite thick.415. and 416. The changes probably result in part from the superadded changes caused by rubbing and scratching. They may be overdiagnosed clinically as squamous cell carcinoma. 415 The acantholytic variant clinically mimics a basal cell carcinoma. It is sometimes present in lesions not responding to local therapy but whether it is the cause of the treatment failure or the result of treatment is speculative. The spreading pigmented actinic keratosis is a brown patch or plaque, usually greater than 1 cm in diameter, that tends to spread centrifugally.417. and 418. Some cases appear to represent the collision of an actinic keratosis and solar lentigo. 419 The cheeks and forehead are sites of predilection. Dermoscopy of the pigmented actinic keratosis has a striking similarity to lentigo maligna.420. and 421. The lichenoid actinic keratosis (not to be confused with the lichen planus-like keratosis) is not usually distinctive, although sometimes local irritation is noted. 422
Cumulative exposure to sunlight appears to be important in the etiology. Intermittent, intense ultraviolet (UV) exposure in childhood, manifest as sunburn, is also strongly associated with the prevalence of actinic keratoses. 423 It has been regarded as an ‘occupational and environmental disorder’. 424 Despite educational programs, a significant number of individuals still experience sunburns. 425 It remains to be seen whether the use of sunless tanning products will result in safer sun protection strategies. 426 Recent work has demonstrated that Betapapillomavirus infection in combination with key risk factors increases the risk of actinic keratoses. 389 These viruses were previously categorized as epidermodysplasia verruciformis (EV) – HPV types. Abnormalities in DNA synthesis in keratinocytes in the skin around the lesion suggest that there is a gradual stepwise progression from sun-damaged epidermis to clinically obvious keratoses, and eventually to squamous cell carcinoma.427. and 428. This occurs at both the morphological and molecular level. 429 The long-term use of hydroxyurea can also produce squamous dysplasia which may be a precursor of multiple squamous cell carcinomas. 430 The keratinocytes in solar keratoses, like those in squamous cell carcinomas, lose various surface carbohydrates. 431
Approximately 50% of actinic keratoses and squamous cell carcinomas show overexpression of cyclin D protein as well as p53 positivity.432.433.434.435.436. and 437. Bcl-2 may be increased in actinic keratoses. 438 The presence of these regulatory markers correlates with the severity of the solar elastosis, suggesting that the grade of solar elastosis is a helpful marker of epithelial UV damage. 439 Tenascin expression is increased in the stroma beneath actinic keratoses and this increases with the atypia. 440 Activated ras genes are found in a small percentage of cases. 441 There is also diminished expression of tumor suppressor genes. 442 However, no genetic susceptibility to actinic keratoses has been found. 436
Treatment guidelines, prepared on behalf of the British Association of Dermatologists, were published in 2007. They are, where possible, evidence based. They are too complex to summarize here as the preferred therapy differs, based on the number of lesions, the type of lesions, response to previous therapies, the location of the lesions, and characteristics of the patient. 443 The therapies listed include cryosurgery,444. and 445. 5-fluorouracil, diclofenac, imiquimod (not licensed for this purpose in some countries), resiquimod, 446 curettage, and photodynamic therapy. 443 Some patients show adverse reactions to diclofenac. 447 Plastic surgeons are more likely to use excisional biopsies and dermatologists shave biopsies to make the diagnosis of actinic keratosis. 448 Photodynamic therapy (PDT) is effective in up to 91% of actinic keratoses in trials comparing it with cryotherapy. Although more expensive, it is particularly useful when multiple keratoses are present.443.449. and 450. However, a randomized trial published just recently found PDT to have inferior efficacy for treatment of multiple actinic keratoses on the extremities when compared with cryotherapy. 451 Stabbing headaches are experienced by some patients receiving cryotherapy for lesions on the face. 452 Another trial has found that continuous activation of porphyrins by daylight is as effective as conventional PDT. 453 Photodynamic therapy results in apoptosis of atypical keratinocytes. 454 Imiquimod cream 5% is another effective treatment,455.456.457. and 458. but the duration of treatment required sometimes results in poor patient compliance. Imiquimod stimulates a cutaneous immune response characterized by increases in activated dendritic cells and CD4+ and CD8+ T cells. 456 It also favorably influences the expression of genes in actinic keratoses, 459 but squamous cell carcinoma may still arise subsequently in a treatment site.460. and 461. Resiquimod, which is more potent than imiquimod, is still undergoing evaluation. 446 Other treatment reviews have been published.382. and 462.
Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia, consistent with its ability to act as a chemopreventive agent in epithelial carcinogenesis.463. and 464. It also thickens the collagen band in the papillary dermis. 464 Facial resurfacing using the carbon dioxide laser, a 30% trichloroacetic acid peel, or 5% fluorouracil cream have been shown to reduce the subsequent development of actinic keratoses and skin cancers compared with a control group. 465
Little has been written on the recurrence rate of actinic keratoses following biopsy with incomplete margins. In one recently published study, of 85 incompletely removed actinic keratoses not subjected to further treatment, 48.2% recurred. All recurred as actinic keratoses except one case, which recurred as a squamous cell carcinoma. 466
Diagnostic biopsy is undertaken in only a small percentage of actinic keratoses diagnosed clinically. 385 The clinical accuracy in the recognition of actinic keratoses varies from 74 to 94%. 468 The usual actinic keratosis is characterized by focal parakeratosis, with loss of the underlying granular layer and a slightly thickened epidermis with some irregular downward buds (Fig. 31.13). Uncommonly, the epidermis is thinner than normal. In all cases there is variable loss of the normal orderly stratified arrangement of the epidermis; this is associated with cytological atypia of keratinocytes, which varies from slight to extreme. The term ‘bowenoid keratosis’ may be used when the atypia is close to full thickness. 417 This variant differs markedly from the ‘de novo’ form of Bowen’s disease (see below). Sometimes the dysplastic epithelium shows suprabasal cleft formation (see below).379.469. and 470. There is often a sharp slanting border between the normal epidermis of the acrotrichia and acrosyringia and the parakeratotic atypical epithelium of the keratosis. 471 However, dysplastic epithelium may involve the infundibular portion of the hair follicle.471.472. and 473. The parakeratotic scale may sometimes pile up to form a cutaneous horn. 379 Large keratohyaline granules are sometimes present in actinic keratoses. 474
Solar keratosis. There is mild to moderate atypia of keratinocytes and some pallor of cells. There is overlying parakeratosis. (H & E)
Actinic keratoses must be distinguished from the epidermal dysmaturation that may be seen following chemotherapy or transplantation. 475 It is a histological diagnosis characterized by disruption of keratinocyte maturation, loss of polarity, widened intercellular spaces, irregular large nuclei, mid-epidermal mitotic figures, and apoptosis.476. and 477. Colchicine intoxication can also result in dysmaturation with metaphase-arrested keratinocytes and basal vacuolar change. 478
The dermal changes include actinic elastosis, which is usually quite severe, and a variable, but usually mild, chronic inflammatory cell infiltrate. 379 As mentioned above, the grade of solar elastosis is a marker of epithelial UV damage. 439 Inflammatory keratoses may develop during chemotherapy of malignant disease with fluorouracil and its analogues.479.480.481. and 482. There is vascular telangiectasia and a moderately heavy mixed inflammatory cell infiltrate in the upper dermis. Inflammation of actinic keratoses has also been reported following therapy with sorafenib, a multitargeted tyrosine-kinase inhibitor. 483 Some actinic keratoses progress to squamous cell carcinoma with this therapy. 483 An inflammatory response is also present in actinic keratoses before they progress to squamous cell carcinomas, unrelated to any therapies. 484 The inflammation subsides rapidly following this conversion. 484
In the hyperplastic (hypertrophic) form there is prominent orthokeratosis with alternating parakeratosis. 415 The epidermis usually shows irregular psoriasiform hyperplasia, and sometimes there is mild papillomatosis. Dysplastic changes are sometimes minimal and confined to the basal layer. 416 The presence of vertical collagen bundles and some dilated vessels in the papillary dermis is evidence that these lesions represent actinic keratoses, with superimposed changes due to rubbing or scratching (lichen simplex chronicus). 415 Hyperplastic (hypertrophic) forms have a higher resistance to apoptotic stimuli compared to atrophic variants. 485
In the closely-related proliferative variant, there is a strong propensity to transform into an invasive squamous cell carcinoma. 486 Nests of atypical cells extend as finger-like projections into the upper dermis. It has a pseudo-infiltrative appearance. Extension down hair follicles and, less frequently, acrosyringia may be present. A heavy inflammatory cell infiltrate of lymphocytes and some plasma cells is often present. 486
In the atrophic actinic keratosis, the epidermis is thin with only several layers of keratinocytes. Atypia is usually limited to the basal layer. There is usually overlying hyperkeratosis and focal parakeratosis.
In the acantholytic variant, there are clefts, usually suprabasal in location, although the change may be more widespread with acantholytic and dyskeratotic cells resulting from anaplastic/atypical changes producing disrupted intercellular bridges. 382
In the pigmented variant there is excess melanin in the lower epidermis, usually in both keratinocytes and melanocytes, but sometimes only in one or the other. 417 Melanophages are usually found in the papillary dermis. 417
The bowenoid actinic keratosis is a controversial entity. It is usually a focal change, indistinguishable from Bowen’s disease. Overlying parakeratosis is often present. The author rarely uses the term these days, and only as a ‘fence-sitting’ diagnosis on 2 mm biopsies (or smaller). The pagetoid variant of actinic keratosis is closely related. 487
In lichenoid actinic keratoses there is a superficial, often band-like, chronic inflammatory cell infiltrate, with occasional apoptotic keratinocytes in the basal layer and some basal vacuolar change. 422 The acral keratotic lesions with a lichenoid infiltrate, reported as a possible manifestation of graft-versus-host disease, may have been a manifestation of HPV infection, as wart-virus features sometimes remit in the presence of a lichenoid infiltrate. 488
The lymphomatoid keratosis is mentioned here for completeness although it does not have epidermal atypia or lichenoid (interface) changes. 489 It is an epidermotropic subtype of cutaneous lymphoid hyperplasia with a dense, band-like infiltrate of lymphocytes beneath a sometimes hyperplastic epidermis. It looks like a lichen planus-like keratosis but with epidermotropism rather than basal cell death. 489
In all types of actinic keratoses in immunosuppressed patients there is usually marked atypia of the keratinocytes; 490 multinucleate forms may be present. 490 Confluent parakeratosis and verruciform changes may also occur.491. and 492.
It is sometimes a matter of personal judgment whether a lesion is considered to show early squamous cell carcinomatous change or not. 493 The protrusion of atypical cells into the reticular dermis and the detachment of individual nests of keratinocytes from the lower layers of the epidermis are criteria used to diagnose invasive transformation. 493 Step sections are important in small biopsies initially regarded as solar keratosis. More significant pathology may emerge in the deeper sections.494. and 495. Despite these difficulties there is a good concordance in the diagnosis of these borderline cases among dermatopathologists. 496 An AgNOR analysis (see p. 723) may identify actinic keratoses with high proliferative activity and an increased tendency to develop into invasive squamous cell carcinoma. 497
Confocal laser microscopic imaging of actinic keratoses has been used. 498 A diagnosis of actinic keratosis can often be made by this technique. 499 Fluorescence techniques are not a suitable method of distinguishing keratinocytic atypias from normal skin. 500
Ultrastructural studies suggest that the hyperpigmentation in the pigmented variant is due to enhanced melanosome formation and distribution, and not to a block in the transfer of melanosomes to keratinocytes. 418
Actinic cheilitis (solar cheilosis, actinic keratosis of the lip) is a premalignant condition seen predominantly on the vermilion part of the lower lip. It results from chronic exposure to sunlight, 501 although smoking and chronic irritation may also contribute. 502 There are dry, whitish-gray scaly plaques in which areas of erythema, erosions, and ulceration may develop. 501 The whitish areas were known in the past as leukoplakia. 503 Large areas of the lower lip may be affected. Squamous cell carcinoma may develop after a latent period of 20–30 years,504. and 505. although the incidence of this transformation is difficult to quantify. 502 This transformation appears to be related to dysregulation of the signal transducer and activator of transcription-3 (STAT-3), a protein involved in the control of cell proliferation and apoptosis. 506
An acute form of actinic cheilitis, characterized by edema, erythema, and erosions, has been recognized. 503 It is an uncommon response to prolonged exposure to sunlight.
Treatment usually parallels that given for actinic keratoses at other sites and includes cryotherapy, electrosurgery, imiquimod, retinoids, carbon dioxide laser ablation, and surgical excision. 507 Photodynamic therapy has also been used successfully. 507
The lesions show alternating areas of orthokeratosis and parakeratosis. The epidermis may be hyperplastic or atrophic. Other features are disordered maturation of epidermal cells, increased mitotic activity, and variable cytological atypia. 504 Sometimes foci of severe atypia are found when the entire vermilion is removed even though the biopsy showed milder changes. 509 Squamous cell carcinoma may develop in areas of marked atypia. Cytokeratin expression in actinic cheilitis is not related to the degree of dysplasia. 510
There is prominent solar elastosis of the submucosal connective tissue, some vascular telangiectasia, and a mild to moderate infiltrate of chronic inflammatory cells. Plasma cells are usually prominent, particularly beneath areas of ulceration. 508 An intense inflammatory cell infiltrate is often predictive of an adjacent invasive squamous cell carcinoma. 511
Actinic cheilitis and Bowen’s disease both need to be distinguished from pagetoid dyskeratosis, which has been found as an incidental histological feature in over 40% of specimens from the lip. 512 It is composed of large keratinocytes with a condensed nucleus, a clear halo, and abundant pale cytoplasm. 512 This change is not confined to the lips (see p. 272).513. and 514.
For more than a century inorganic arsenic was used in the treatment of many diverse conditions. 515 The recognition of its adverse effects, and its replacement by more effective therapeutic agents, has led to a marked reduction in the incidence of arsenic-related conditions. 516 However, there is a high arsenic content in some drinking waters and naturopathic medicines.517.518.519.520.521.522. and 523. Chronic arsenic poisoning is a worldwide public health problem. 524
The best-known effect of chronic arsenicism is cutaneous pigmentation, which may be diffuse or of ‘raindrop’ type. 525 More than 40% of affected individuals develop keratoses on the palms and soles, and sometimes this is associated with a mild diffuse keratoderma. 525 Hyperkeratosis on the sole is the most sensitive marker for the detection of arsenicism at an early stage. 526 There is an increased incidence of multiple skin cancers, which include Bowen’s disease, basal cell carcinomas, and squamous cell carcinomas.521.527. and 528. The lesions are sometimes quite exophytic in appearance. Visceral cancers, particularly involving the lung and genitourinary system, may also be found. 529
Many arsenical skin cancers express p53, although arsenic-related basal cell carcinomas express it less intensely than sporadic ones. 530 It is also found in perilesional skin.531. and 532. The expression of p53 is reduced after UVB therapy. 533 Arsenic also appears to produce defective expression of β1-integrins in arsenical keratoses. 534
Arsenical keratoses are of the hyperkeratotic type. Sometimes there is prominent hyperkeratosis and papillomatosis, but no atypia. These lesions have a superficial resemblance to the hyperkeratotic type of seborrheic keratosis. Similar lesions follow exposure to tar (Fig. 31.14). 535 In other cases there is mild atypia resembling the hyperkeratotic variant of actinic keratosis.
Tar keratosis. There is pronounced hyperkeratosis, papillomatosis and acanthosis. (H & E)
In some lesions of Bowen’s disease related to exposure to arsenic there may be areas resembling seborrheic keratosis, superficial basal cell carcinoma, or intraepidermal epithelioma of Jadassohn. Invasive carcinomas arising in Bowen’s disease show the non-keratinizing pattern of squamous cell carcinoma, sometimes with areas of appendageal differentiation (see below).
The basal cell carcinomas that develop may be of solid or (multifocal) superficial type.
A PUVA keratosis is a distinctive form of keratosis, often found on non-sun-exposed skin of patients who have received long-term treatment with psoralens and ultraviolet-A radiation (PUVA). 536 It is a raised papule with a broad base and a scaly surface, often with a warty appearance. There is an increased risk of developing non-melanoma skin cancer, particularly with long-term, high-dose exposure.537.538. and 539. Punctate keratoses on the hands and feet are a rare complication of PUVA therapy. 540
There is a variable degree of acanthosis, orthokeratosis, and parakeratosis. Papillomatosis is present in one-half of the lesions. PUVA keratoses differ from actinic keratoses by their paucity of atypical cells and an absence of solar elastosis. 536 The lesions reported as disseminated hypopigmented keratoses developed in young patients who had previously received PUVA therapy. 541 They had some histological resemblance to stucco keratoses (see p. 701). 541
Although the term ‘intraepidermal carcinoma’ is often used synonymously with Bowen’s disease, it is used here in a broader sense to include not only carcinoma in situ of the skin (Bowen’s disease), and penis (erythroplasia of Queyrat), but also intraepidermal epithelioma of Jadassohn, a controversial entity of disputed histogenesis. Paget’s disease is sometimes included in this category because of the presence of cytologically malignant cells within the epidermis. Paget’s disease is discussed with the appendageal tumors on page 788.
Bowen’s disease is a clinical expression of squamous cell carcinoma in situ of the skin. 542 It presents as an asymptomatic well-defined erythematous scaly plaque, which expands centrifugally. Verrucous, nodular, eroded, and pigmented variants occur.543.544.545.546. and 547. Many of the pigmented lesions reported in the anogenital area as Bowen’s disease548.549. and 550. would now be regarded as examples of bowenoid papulosis551 (see p. 625).
Bowen’s disease has a predilection for the sun-exposed areas (particularly the face and legs) of fair-skinned older individuals.552.553. and 554. It is uncommon in black people, 555 in whom it is found more often on areas of the skin that are not exposed to the sun. 556 Its incidence in the Canadian province of Alberta, in the 5-year period from 1996 to 2000, was 22.4 lesions per 100 000 women and 27.8 per 100 000 men. 557 Lesions may also develop on the trunk, 558 and the vulva, and rarely on the nail bed,559.560.561.562.563.564.565. and 566. where it may be polydactylous, 567 lip, 568 nipple, 569 palm,570.571. and 572. sole, 573 web-spaces of the feet, 574 and the margin of an eyelid.575. and 576. In one case Bowen’s disease of the nail bed presented as a longitudinal melanonychia. 577 In another there was periungual Bowen’s disease and vulval intraepithelial neoplasia (VIN) concordant for the same HPV types (HPV-34 and HPV-21). 578 Bowen’s disease has been reported in the wall of an epidermoid, and a follicular cyst,579. and 580. in a lesion of porokeratosis of Mibelli, 581 above a scar, 582 in erythema ab igne, 583in a smallpox vaccination scar, 584 and in seborrheic keratoses (see p. 672).
Several investigators have proposed that Bowen’s disease should be considered a skin marker for internal malignant disease,585.586.587.588. and 589. although more recent studies have shown no evidence for this association.590.591. and 592. However, patients with Bowen’s disease have the same increased risk of developing a subsequent skin cancer as do those with invasive squamous cell carcinoma. 593
Invasive carcinoma develops in up to 8% of untreated cases.594. and 595. This complication, which is not well recognized, is characterized by the development of a rapidly growing tumor, 1–15 cm in diameter, in a pre-existing scaly lesion. 595 It appears to be more common in older people, and in immunocompromised patients. 596 Invasive squamous cell carcinoma is a not uncommon complication of the diffuse variant of Bowen’s disease, associated with extensive adnexal extension of the disease. 597 The invasive tumor has metastatic potential, which has been stated to be as high as 13%, 595 although this would appear to be an overestimation of the risk.594.598.599. and 600. Spontaneous complete regression of Bowen’s disease has also been reported. 601
Several factors have been implicated in the etiology of Bowen’s disease. They include prolonged exposure to solar radiation, the ingestion of arsenic,525.585. and 602. and infection with the human papillomavirus (HPV). 603 Whereas HPV type 16 (HPV-16) and HPV-18, and to a lesser extent HPV-18, 31, 33, 39, 52, 67, and 82 have been detected in Bowen’s disease of the genital region and its precursors,604.605. and 606. there are now several reports of non-genital Bowen’s disease related to infections with HPV-2, 607 HPV-16,608.609.610.611. and 612. HPV-27, 613 HPV-33, 613 HPV-34,614. and 615. HPV-56,616. and 617. HPV-58, 618 HPV-59, 619 and HPV-76. 613 Epidermodysplasia verruciformis-related HPV types (Betapapillomavirus) are associated with the pathogenesis of Bowen’s disease as well as the better known mucosal strains of HPV. 620 There is a clear association between anal intraepithelial neoplasia and high-risk HPV types in patients with HIV infection.621. and 622. It is an important precursor of invasive squamous cell carcinoma.623. and 624. Bowen’s disease is a rare complication of the treatment of psoriasis with psoralens and ultraviolet-A radiation (PUVA).625.626. and 627. It has been reported in a patient who performed arc welding. 628
Dermoscopy can be helpful for diagnosing Bowen’s disease. It is characterized by glomerular vessels and a scaly surface. 629 In pigmented lesions, small brown globules and/or homogeneous pigmentation are also present. 629
Bowenoid papulosis (see p. 625) consists of one or more indolent, verrucous papules on the genitalia with a clinical resemblance to condyloma acuminatum and a histological resemblance to Bowen’s disease. HPV-16 is the most frequently detected HPV subtype detected in this condition. 630 It usually responds to local therapies, but recurrences and the development of invasive carcinoma have been reported. 605 The term ‘penile intraepithelial neoplasia’ (PIN) has been coined to encompass the three preinvasive clinical entities of penile Bowen’s disease, erythroplasia of Queyrat, and bowenoid papulosis. 631
No single therapeutic option is unequivocally superior to any other and the treatment used often depends on patient preference, cost, the clinical situation, and the clinician’s experience/preference for a particular therapy.632.633.634.635.636.637. and 638. Cryotherapy is most commonly used. Recurrences occur particularly when adnexal structures are involved. Curettage ± cautery gives cure rates of 93–98%. 638 It is also cost effective. 639 Imiquimod cream 5% produces clearance in about 80% of cases.640.641.642.643. and 644. Invasive squamous cell carcinoma has developed in several cases treated with this therapy. 645 Some patients discontinue imiquimod because of a local reaction.
Photodynamic therapy, usually in conjunction with topical 5-aminolevulinic acid, is effective for large or multiple lesions.646. and 647. It has also been used for subungual lesions. 648 Extensive vulval intraepithelial neoplasia (VIN) has been treated using photodynamic therapy with meta-tetrahydroxychlorin. 649
Radiation therapy is now used less often. 650 Acitretin has been used to treat multiple arsenical keratoses and Bowen’s disease. 651 Surgery is often used. Recurrence rates are usually less than 5%. 557 A 5-year recurrence rate of 6.3% was obtained in 270 cases treated with Mohs surgery, but the majority of cases were on the head and neck where standard surgery is associated with higher recurrence rates. 652
Bowen’s disease is a form of carcinoma in situ, and accordingly shows full-thickness involvement of the epidermis, and sometimes the pilosebaceous epithelium, by atypical keratinocytes.654.655. and 656. This is associated with disorderly maturation of the epidermis, mitoses at different levels, multinucleate keratinocytes, and dyskeratotic cells. Usually there is loss of the granular layer, with overlying parakeratosis and sometimes hyperkeratosis.
Several histological variants have been described, and more than one of these patterns may be present in different areas of the same lesion. 653 In the psoriasiform pattern there is regular acanthosis with thickening of the rete ridges and overlying parakeratosis (Fig. 31.15). 653 In the atrophic form there is thinning of the epidermis, which shows full-thickness atypia and disorganization. 653 There is usually overlying hyperkeratosis and parakeratosis. The verrucous-hyperkeratotic type is characterized by hyperkeratosis, papillomatosis, and sometimes intervening pit-like invaginations. 653 The papillated variant, which has sometimes been included with the verrucous-hyperkeratotic type, is an exophytic/endophytic, sometimes keratotic lesion. 657 A perinuclear halo is present in some of the cells suggestive of koilocytosis. 657 The irregular variant shows irregular acanthosis, and often extensive chronic inflammation in the underlying dermis. 653 In the pigmented type there is melanin in individual tumor cells and melanophages in the underlying dermis. 551 The pagetoid variant has nests of cells with pale cytoplasm and thin strands of relatively normal keratinocytes intervening; the basal layer may also be spared (Fig. 31.16). Sometimes this is associated with psoriasiform hyperplasia of the epidermis. Mucinous and sebaceous metaplasia characterize two other rare histological patterns. 658Clear cell change represents outer root sheath differentiation. 659 HPV types have been identified in this clear cell variant. 660
Bowen’s disease. There is full-thickness atypia of the epidermis, which also shows psoriasiform hyperplasia. (H & E)
Bowen’s disease. The atypical keratinocytes are pale, with a pagetoid appearance. (H & E)
As already mentioned, the atypical epithelium may also involve the pilosebaceous units. This may lead to treatment failure when superficial methods of destruction are used.661. and 662. Involvement of the eccrine ducts is uncommon,663. and 664. and in the author’s experience it has usually been confined to cases of Bowen’s disease of the temple region. 665
Changes in the underlying dermis include increased vascularity and a variable inflammatory response, which is usually composed of lymphocytes. Occasionally this has lichenoid features. Partial regression may ensue. 666 Small deposits of amyloid may be found in the papillary dermis, particularly in lesions of long standing. 653
The pagetoid variant of Bowen’s disease is sometimes difficult to distinguish from Paget’s disease and from in-situ superficial spreading melanoma, particularly if only a small biopsy is available.667. and 668. In these instances immunoperoxidase markers may be of assistance. Melanoma cells are positive for S100 protein, whereas Paget cells usually demonstrate carcinoembryonic antigen (CEA). 669 Melanoma cells do not contain cytokeratins, although Paget cells are positive for cytokeratins with a molecular weight of 54 kDa and negative for those of 66 kDa; the reverse applies with the cells in Bowen’s disease. 670 Although cytokeratin 7 (CK7) has been regarded as a specific marker of Paget’s disease, it has also been reported in pagetoid Bowen’s disease, but not in the other types. 671 In Bowen’s disease there is a diffuse pattern of staining of the keratinocyte nuclei for PCNA (proliferating cell nuclear antigen).672.673.674. and 675. There is widespread expression of CK10 in almost all cases of Bowen’s disease. 676 CD1a+ cells are significantly decreased. 677 In clear cell Bowen’s disease the cells express keratins found in the outer root sheath, such as CK13, CK16, and CK15. The cells stain with CAM5.2. 659 The expression of CK14 may be a marker for tumor progression. 678
Although p16 overexpression is a surrogate marker of HPV E7-mediated catabolism of the regulatory protein pRb in premalignant and malignant lesions of cervical mucosa, its overexpression in cutaneous Bowen’s disease is unrelated to HPV status. 679 Its overexpression in Bowen’s disease may reflect disruption of the G1/S checkpoint, resulting in unregulated cell cycle progression.679. and 680.
In the invasive form there are large islands of non-keratinizing squamoid cells throughout the dermis. 595 The cells usually have pale cytoplasm. Basaloid and adnexal differentiation are common patterns.595.681. and 682. The invasive tumor that supervenes is best regarded as a variant of squamous cell carcinoma. Invasion may be facilitated by the production of metalloproteinases which are involved in the destruction of basement membrane. 683
The term ‘Bowen’s disease’ is no longer used in gynecological pathology, having been replaced in 1986 by the concept of vulvar intraepithelial neoplasia (VIN).684.685.686. and 687. Progressive atypia of the epithelium is semiquantified, VIN I representing atypia confined to the basal third of the epidermis, and VIN II corresponding to involvement of from one-third to two-thirds of the epithelium. In VIN III, the atypical cells involve more than two-thirds of the thickness of the epidermis. Bowen’s disease therefore corresponds to severe VIN III. 688 There has been an attempt to apply this concept, in part, to other areas of the skin. 689
In 2004, the International Society for the Study of Vulvovaginal Disease abandoned the grading of vulvar intraepithelial neoplasia. Little has been published in the dermatological or pathological literature on this change.690. and 691. The old VIN I is regarded now as representing HPV infection while VIN II and VIN III have been combined to form a warty-basaloid type of VIN to distinguish it from the less common differentiated VIN which remains unchanged in the new nomenclature. 690 While squamous cell carcinoma in situ is regarded as an acceptable alternative designation to VIN, Bowen’s disease is not, because it is an eponymous designation with ‘no intrinsic definition’. 690 Risk factors associated with various vulvar intraepithelial lesions were reviewed in 2005. 692
‘Vulvar intraepithelial neoplasia’ should be distinguished from the recently described entity multinucleated atypia of the vulva, in which cells with 2–10 nuclei are found in the cells of the lower layers of the epithelium of the vulva. 693 The cells lack hyperchromasia or variation in nuclear size. HPV has not been detected. The nature of this process is uncertain. Parenthetically, the author has seen multinucleate cells in the epidermis of patients who have applied retinoic acid for a long time (unpublished observation).
Rare cases have been reported of metastatic squamous cell carcinoma presenting as epidermotropic bowenoid lesions. 694
Bowen’s disease differs from actinic (solar) keratosis in the full-thickness atypia of the epithelium and in usually sparing the acrosyringium and sometimes also the basal layer which is always atypical in actinic keratoses. Both lesions may show aneuploidy of the constituent cells, 695 and both may express mutant p53 protein and p21.696.697. and 698. In solar keratoses the keratin and involucrin distribution is similar to normal epidermis, whereas in Bowen’s disease the keratin distribution is variable. 699 In arsenical keratoses, in-situ carcinoma indistinguishable from Bowen’s disease may develop. Pagetoid dyskeratosis (see p. 272) differs from Bowen’s disease by the presence of scattered pale cells with small pyknotic nuclei in a background of otherwise normal skin. 513
Attempts have been made to introduce a three-tiered grading system for keratinocytic atypia, similar to the previous system of grading atypia on the vulva and cervix. In an informal trial, dermatopathologists were reliably able to categorize the continuum of keratinocytic atypia with substantial concordance.700.701. and 702. Keratinocytic intraepithelial neoplasia (KIN) has not yet received much acceptance in the broader community of pathologists and dermatologists. The concept is not supported by experimental work involving cyclin A levels in actinic keratosis and Bowen’s disease. 703
Bowenoid papulosis of the genitalia is regarded by some as a variant of Bowen’s disease of the genitalia; although the two conditions may be histologically indistinguishable, features that favor a diagnosis of bowenoid papulosis include numerous mitoses in metaphase, small basophilic inclusions in the cytoplasm of the granular layer, and the presence sometimes of cells with a resemblance to koilocytes (Fig. 31.17).
Bowenoid papulosis. There is full-thickness atypia, scattered mitoses in metaphase, basophilic inclusions in the granular layer, and koilocytes. (H & E)
The keratinocytes have large nuclei and nucleoli, and a reduced number of desmosomal attachments. 705 The dyskeratotic cells show an aggregation of cytoplasmic tonofilaments. Occasional apoptotic bodies are present in the intercellular spaces, whereas others have been phagocytosed by neighboring keratinocytes.706. and 707. Cytoplasmic projections of keratinocytes may extend through gaps in the basement membrane. 704
Erythroplasia of Queyrat is a clinical expression of carcinoma in situ of the penis.708.709.710.711.712. and 713. It is found most commonly on the glans penis of uncircumcised males as a sharply circumscribed, asymptomatic, bright red shiny plaque. 710 It may also arise on the coronal sulcus or the inner surface of the prepuce. As in Bowen’s disease, invasive carcinoma may develop in up to 10% of cases of erythroplasia of Queyrat, and such tumors have metastatic potential. 708
The etiology of this condition has been regarded as multifactorial: chronic irritation, poor hygiene, genital herpes simplex, and infection with human papillomavirus (HPV) have all been incriminated. 714 It has also arisen on a background of Zoon’s balanitis. 715 Interestingly, the patient had been treated with topical pimecrolimus a few weeks before the appearance of the lesion. 715 In one study, the rare epidermodysplasia verruciformis-associated subtype, HPV-8, was detected in all cases studied. 716 A co-infection with other HPV types, particularly HPV-16, HPV-39, and HPV-51, was usually present. 716
Imiquimod 5% cream, topical 5-fluorouracil, cryotherapy, and photodynamic therapy have all been used to treat this condition.638.715.717. and 718.
The intraepidermal epithelioma (Jadassohn) has been regarded by some as a distinct clinicopathological entity, characterized by the presence of nests of atypical keratinocytes within the epidermis and having the potential to progress to invasive squamous cell carcinoma in a small number of cases.720.721. and 722. As defined, it presents as a scaly plaque, usually on the lower part of the trunk, the buttocks or the thighs, measuring from 0.5 to 10 cm in diameter. 721
Most authors do not recognize the existence of such an entity, which they believe is simply an expression of the Borst–Jadassohn phenomenon, a term used to describe the presence of sharply defined nests of morphologically different cells within the epidermis.723. and 724. This phenomenon may be seen in seborrheic keratoses (clonal variant), hidroacanthoma simplex and some cases of Bowen’s disease, actinic keratosis, and, rarely, epidermal nevi.725. and 726. It has also been seen in a tumor in which the nests of cells exhibited markers for hair follicle cells by immunoperoxidase: accordingly, the lesion was called an intraepidermal pilar epithelioma. 727
This group, which includes basal cell and squamous cell carcinomas, accounts for approximately 90% or more of all skin malignancies. These tumors constitute an important public health problem,728.729. and 730. despite their comparatively low mortality rate.731.732. and 733. The lifetime risk for the development of skin cancer in the United States is 1 in 5. 734 It was estimated that over two million cases of non-melanoma skin cancer (NMSC) were diagnosed in the United States in 2004. 735 In Germany between 1998 and 2001, the age-standardized rate for all NMSC was 100.2 per 100 000 inhabitants per year for men and 72.6 for women, with 80% of all tumors being basal cell carcinoma. 736 In Australia, it was estimated that in 2002, there were 374 000 new cases of basal cell carcinoma and squamous cell carcinoma. 737 In most instances the histopathological diagnosis is straightforward, but very occasionally tumors are encountered which are difficult to classify because of some apparent morphological overlap with various appendageal tumors, or because the tumor exhibits both basaloid and squamous differentiation. In these circumstances immunohistochemistry may be of assistance. 738
In a review of lethal non-melanoma skin cancers in Western Australia, 120 cases were recorded during a 5-year period. Of these cases, 89 were caused by squamous cell carcinoma, 22 by Merkel cell carcinoma, and four were adenosquamous carcinomas. 739 The mortality increases sharply with age. 740 A similar study in Danish patients found markedly different mortality rates between squamous cell carcinomas and basal cell carcinomas (in which it was very low). 741
Basal cell (trichoblastic) carcinomas are the most common cutaneous tumors, accounting for approximately 70% of all malignant diseases of the skin. 742 They exceed squamous cell carcinomas in frequency by a factor of approximately 5:1, although this ratio varies from 2:1 to 7:1 in different latitudes.743.744.745.746. and 747. The age-adjusted incidence per 100 000 varies from 106 in Canada to 980 in non-Hispanic white persons in south-eastern Arizona to 1193 in Townsville, North Queensland, Australia.748. and 749. The incidence in northern Europe is lower than in Canada. 750 Of course, if solar keratoses are regarded as squamous cell carcinomas (see p. 693) then squamous cell carcinoma becomes more common than basal cell carcinoma.751. and 752. The overall incidence of basal cell carcinomas appears to be increasing, 753 particularly in males over the age of 70 years. 754 There is some evidence of a reduction in incidence in younger birth cohorts. 736
Because of the undoubted links between basal cell carcinoma and a follicular origin, there have been calls for it to be renamed trichoblastic carcinoma, 755 and inferences that it should not be included as a tumor of the epidermis. 756 Major name changes for extremely common conditions are not well accepted by the general readership or the medical profession. While acknowledging the evidence of follicular differentiation and follicular markers in basal cell carcinomas, the author does not propose changing the title or textual location of this entity. Changes of this magnitude need to be evolutionary rather than confronting.
Basal cell carcinomas are found predominantly on areas of skin exposed to the sun, particularly in fair-skinned individuals.757.758.759. and 760. They are rare in black people.761.762.763. and 764. Up to 80% of all lesions are found on the head and neck,743.765. and 766. whereas approximately 15% develop on the shoulders, back, or chest. 767 There are isolated reports documenting involvement of the lacrimal caruncle, 768 vermilion lip, 769 breast, 770 nipple,771. and 772. axilla,773. and 774. perianal region,775.776.777. and 778. vulva,778.779.780.781.782. and 783. penis,778.784.785. and 786. scrotum,778.787.788.789.790. and 791. inguinal region, 792 subungual skin and nail unit,793.794.795.796. and 797. lower part of the legs, 798 and the palms792.799. and 800. and soles.801.802. and 803. Other unusual sites of involvement include pilonidal sinuses, 804 venous ulcers,805. and 806. sternotomy scars, 807 an acupuncture site, 808 the skin overlying arteriovenous malformations,809. and 810. the nose affected by rhinophyma,811. and 812. and the scars that follow thermal burns, 813 radiation,814.815. and 816. chickenpox, 817 leishmaniasis,818. and 819. smallpox, 820 and influenza, 821 and BCG vaccination.822. and 823. Basal cell carcinomas develop in approximately 20% of organoid nevi,824.825. and 826. and, rarely, in epidermal nevi,22. and 827. fibroepithelial polyps, 828 multiple trichoepitheliomas, 829 epidermal cysts, 830‘port wine’ stains,831.832.833. and 834. and solar lentigos. 835 A basal cell carcinoma has been reported in association with granulocytic sarcoma of the skin. 836 Multiple basal cell carcinomas may develop in the basal cell nevus syndrome (see p. 691), and in the rare Bazex’s syndrome (Bazex–Dupré–Christol syndrome – OMIM 301845), in which there is also follicular atrophoderma and hypohidrosis.837.838.839. and 840. It maps to Xq24–q27. They also occur in ROMBO syndrome (OMIM 180730) which includes vermiculate atrophoderma. 841 Multiple basal cell carcinomas have also been reported in the cartilage hair hypoplasia (McKusick) syndrome (OMIM 250250) due to a mutation in the RMRP gene. 842 Furthermore, any patient who has had one basal cell carcinoma has a high probability of subsequently developing a further lesion.843. and 844. Patients with truncal lesions and those presenting with tumor clusters represent a high susceptibility group for the development of further lesions.735.845.846.847. and 848. Tumors in this site are commonly of the (multifocal) superficial type with a male predominance. 849 There appears to be no risk for the development of non-cutaneous cancers. 850 It appears that different mechanisms determine the development of truncal and non-truncal basal cell carcinomas. 851 On the other hand, patients with lesions of the nasolabial fold seem to present with shorter evolution time from first symptom, have a smaller size, less aggressive histological features, but require more complex reconstructions to remove them. 852
Basal cell carcinomas are more common in males, presumably related to occupational and recreational exposure to ultraviolet light. They tend to occur in older people, although they have also been documented in children853.854.855. and 856. and young adults. 857 In children there is often a clinical association with the basal cell nevus syndrome, Bazex’s syndrome, xeroderma pigmentosum, or an organoid nevus. 853
The clinical presentation of a basal cell carcinoma can be quite variable. It may be a papulonodular lesion with a pearly translucent edge, an ulcerated destructive lesion (‘rodent ulcer’), a pale plaque with variable induration, an erythematous plaque with visible telangiectasia, or a partly cystic nodule.858. and 859. Lesions presenting as a large pore may show follicular differentiation microscopically.860. and 861. Giant lesions up to 20 cm in diameter,862.863.864.865. and 866. and variants with mutilation of the face have also been documented.867. and 868. Rare linear and polypoid forms have been reported.869. and 870. Approximately 2–5% of lesions are pigmented; basal cell carcinomas in black people and in the Japanese are often pigmented.763.871. and 872. Rarely, these pigmented variants mimic a malignant melanoma873 or develop a depigmented halo. 874 The confocal microscopy of pigmented basal cell carcinomas is distinctive.875.876. and 877. Dendritic melanocytes can be easily detected by this technique. 878 It is also a highly sensitive method of diagnosing basal cell carcinomas of all types.879.880. and 881. Despite the marked variability in their appearance, the accuracy rate in the clinical diagnosis of basal cell carcinomas is still 60–70%.882. and 883.
Although most basal cell carcinomas are slow-growing, relatively non-aggressive tumors that are cured by most methods of treatment, a minority have an aggressive behavior with local tissue destruction and, rarely, metastasis. 884 Aggressive subtypes have been linked with chronic sun exposure. 885 These aspects will be considered in further detail after the histopathology has been discussed.
Although the prime etiological factor in the development of basal cell carcinoma is exposure to ultraviolet light, particularly the UVB wavelengths, solar dosimetry studies show a poor correlation between tumor density and ultraviolet dose.886.887. and 888. Indeed, susceptibility to UVB-induced inhibition of contact hypersensitivity appears to be a better indicator of cancer risk than cumulative sun exposure, suggesting an important role for immune surveillance in protecting against the development of basal cell carcinoma. 889 Parenthetically, the sun-protection message may be ‘getting through’ to some members of the community. There are now calls for the use of supplemental vitamin D to counteract declining levels of this vitamin in sun-protected individuals. 890 Tumor necrosis factor (TNF) may be an important mediator of the immunosuppression produced by UVB. 889 Various mediators of UVB-induced damage are now being reported.891. and 892. Recreational sunlight exposure in adolescence and childhood appears to be a risk factor for the development of basal cell carcinoma.893.894.895. and 896. Tanning bed exposure may also be a contributory factor. 897 Basal cell carcinomas of the trunk are related to the number of reported sunburns earlier in life. 898 Intermittent exposure may be more important than chronic exposure as clinical actinic elastosis appears to be protective against sporadic lesions. 899 UVB radiation produces DNA damage at mutation hot spots on the p53 tumor suppressor gene. Approximately 50% of all basal cell carcinomas studied have mutations of this gene.900. and 901. Telomerase activation appears to be important in the pathogenesis of basal cell carcinomas. 902 Whereas squamous cell carcinomas tend to develop at the sites of direct exposure to sunlight (dorsum of hands, ears, bald scalp, and lower lip), basal cell carcinomas are more common in sites slightly removed from this, such as the paranasal region and inner canthus. 903 Other predisposing factors include radiotherapy,904.905.906.907.908. and 909. exposure to tar and asphalt, 910 arsenical intoxication,523. and 911. adjuvant treatment of melanoma with isolated limb perfusion, 912 HPV infection,865. and 913. welding, 914 and stasis dermatitis of the legs.915.916. and 917. There is no association with diet, 918 but an inverse association between tea consumption and skin carcinogenesis was found in one study. 919 In females, the risk of developing a basal cell carcinoma increases with the increasing number of nevocellular nevi. 920 Smoking appears to be a risk factor in females for contracting basal cell carcinoma. 897 This is based in part, on a study of twin pairs discordant for cutaneous tumors. 921 It has been suggested that smoking may play a role in inducing sclerosing variants. 922 Tumors have also developed following PUVA therapy in patients with psoriasis. 923 Ultraviolet-A radiation, given to healthy volunteers, has produced decreased epidermal antigen-presenting cell activity which can be blocked by sunscreens. 924 Accordingly, it seems that UVA may contribute to UV-induced immunosuppression. 924
Basal cell carcinomas and squamous cell carcinomas occur in renal transplant recipients,925.926.927. and 928. and in other circumstances of immunosuppression, such as malignant lymphoma or leukemia,929.930.931. and 932. and the AIDS-related complex.933.934.935. and 936. In organ transplant recipients the estimated incidence of non-melanoma skin cancer at 20 years after transplantation is 40–75%. 937 The most critical risk factor is excessive ultraviolet radiation, particularly UVB irradiation. HPV and genetic factors also play a role. 938 Tumors are more aggressive, commence at a younger age, and are more numerous in these circumstances. Squamous cell carcinomas are increased more than basal cell carcinomas, but the ratio of the two tumors still favors basal cell carcinoma. 939 This is contrary to other reports that have found a reversal of the ratio and an SCC/BCC ratio of 3:1.940. and 941. Basal cell carcinomas in solid organ transplant recipients tend to be more frequent in extracephalic sites, and to be superficial in type. 942 They are strongly associated with sun exposure during childhood. 943
There is increasing evidence that genetic factors play a role in the susceptibility of some individuals to basal cell carcinoma. 888 Mutations in the patched homologue 1 gene (PTCH1), which is known to be responsible for the nevoid basal cell carcinoma syndrome (see below), have also been reported in sporadic cases of basal cell carcinoma.947.948.949.950. and 951. PTCH1 mutations have been found in 30–40% of sporadic basal cell carcinomas. 952 Mutations in the PTCH1 gene, the receptor of the sonic hedgehog, have downstream effects leading to the accumulation of the transcription factor Gli-1, which may play a role in the development of basal cell carcinomas.953.954.955. and 956. SOX9, a downstream target of the sonic hedgehog pathway, is expressed in all basal cell carcinomas as well as in adnexal neoplasms of the skin. 957 It is absent in Bowen’s disease and Merkel cell carcinoma. This suggests a possible contribution of SOX9 to the pathogenesis of basal cell carcinomas. Mutations in other genes in the sonic hedgehog pathway have also been found in sporadic tumors.958. and 959. BMI-1, a gene belonging to the polycomb group of epigenetic gene silencers and which is up-regulated by genes in the sonic hedgehog pathway, is overexpressed in basal cell carcinomas. 960 Other unrelated genes have also been implicated.961.962. and 963. Various chemokines may contribute to tumor progression. 964 There is also an association with HLA-DR7 and HLA-DR4 in some populations.965. and 966. Mutations in the BAX gene (bcl-2 associated X protein) and P53 gene have also been found in sporadic cases.967.968. and 969. There is no association between a common polymorphism in the MDM2 gene, a negative regulator of the p53 tumor suppressor, and basal cell carcinoma.970.971. and 972. Loss of heterozygosity on chromosome 4q32–q35 has also been reported in sporadic cases, implicating the p33ING2/ING1L and SAP30 genes. 973
Basal cell carcinomas usually arise from the lowermost layers of the epidermis, although a small percentage may originate from the outer root sheath of the pilosebaceous unit.886. and 974. Whatever their origin – lower epidermis or follicle – the cells in the basal cell carcinoma have many features in common with follicular epithelium, particularly follicular matrix cells, rather than follicular bulge cells as once thought. 975 There is a virtually identical cytokeratin pattern in basal cell carcinomas, trichoblastomas, and developing fetal hair follicles, compelling evidence for a common histogenetic pathway.976. and 977. Another study has found a common expression pattern for epithelial cell adhesion molecule in basal cell carcinoma, early follicular embryogenesis, secondary hair germ, and the outer root sheath of the vellus hair follicle. 756 As already stated, Ackerman now classifies basal cell carcinomas as trichoblastic carcinomas. 755 Further circumstantial evidence of this shared antigenicity is the finding of T lymphocytes in the upper portion of hair follicles, adjacent to a regressing basal cell carcinoma. 978 In contrast to squamous cell carcinomas, basal cell carcinomas are difficult to produce experimentally in animals, although they have been produced in rats using chemical carcinogens. 979 Human lesions can, however, be transplanted to nude mice, but only if the animals are athymic and lacking in natural killer cell activity.933. and 980. Basal cell carcinomas are stroma dependent, and autotransplantation is unsuccessful if the stroma is not included. 981 This stromal dependency is the most likely reason for the low incidence of metastasis of these tumors. 886 Fibroblasts in basal cell carcinomas differ from normal fibroblasts in several ways; they appear to have a regulatory role in the biology of basal cell carcinoma. 982
An update of the previous guidelines for the management of basal cell carcinoma, produced by the British Association of Dermatologists, has recently been published. 983 These guidelines present evidence levels for the various treatments used. They complement the 2006 review of the advantages and disadvantages of the various treatments for this tumor. 984 Surgical excision is the preferred method of treatment for basal cell carcinoma. The experience of the operator is important in getting clear margins.985.986. and 987. Incomplete excision occurs in up to 10% of cases, 988 although higher rates occur, particularly on the mid face. 989 Follow-up of all cases of treated basal cell carcinoma is expensive, but the risk of developing a new basal cell carcinoma after an excision is 11.6% in the first year. 990
Mohs surgery is the treatment of choice for primary facial basal cell carcinomas with aggressive histopathology, and for recurrent tumors on the face. 991 A European study concluded that Mohs surgery was not cost-effective for primary basal cell carcinoma but that it may be for recurrent lesions.992. and 993. The 5-year-recurrence rate for primary tumors treated by this method varies from 1.4 to 3.2% for primary lesions and from 4 to 6.7% for recurrent tumors.991.994. and 995. Another study has found that the difference in recurrence rates between Mohs and conventional surgery was not statistically different. 996 Mohs surgery is effective for the basosquamous carcinoma with a 5-year recurrence rate of 4.1% in one series. 997 There is a high recurrence rate after Mohs surgery in patients with chronic lymphoid leukemia who develop basal cell carcinoma. 998 Aneuploidy is also a risk factor for recurrence. 999 The extent of a basal cell carcinoma can be determined prior to surgery by 5-aminolaevulinate-induced fluorescence.1000.1001. and 1002. Fluorescence lifetime imaging is another method that distinguishes basal cell carcinoma from surrounding uninvolved skin. 1003 Its extent during surgery can be determined by confocal laser scanning of the fresh specimen. 1004 The extent of inflammation in a lesion does not mask tumor nests during Mohs surgery. 1005 Tumor curettage can be used prior to Mohs surgery. 1006
Curettage and cautery (electrodesiccation) is a good treatment for low-risk basal cell carcinomas. 983 For non-aggressive lesions, curettage alone has a cure rate similar to that for curettage and cautery. A 5-year cure rate of 96% has been obtained. 1007 Tumors involving more than 50% of the deep edge of the shave specimen have a high recurrence rate. 1007 The high cure rate even applies to facial lesions. 1008
Cryosurgery is good therapy for low-risk lesions.983. and 1009. It usually gives good cosmetic results. It is the method favored by many family physicians. 1010
Photodynamic therapy (PDT) is another good treatment for low-risk tumors.983.1011.1012. and 1013. Prior curettage is sometimes used. 1014 It may be used for lesions of the central face with good cosmetic results. 1015 It is less effective for pigmented variants than for other types. 1016 Recurrence rates increase with prolonged follow-up. Dermal inflammation and apoptosis occur several days after PDT.1017. and 1018.
Topical imiquimod is effective in most cases of non-infiltrating basal cell carcinoma, particularly superficial lesions. It may leave a hypopigmented macule in many cases. 1019 There is a high clearance rate post-treatment but at 2 years this was only 82% in one study. 1020 It is a useful treatment method in patients with numerous lesions such as the nevoid basal cell carcinoma syndrome, 1021 and in transplant recipients, but only for superficial lesions. 1022 Using curettage prior to imiquimod produced clearance in 32 of 34 (94%) patients. 1023 Imiquimod acts through the Toll-like receptor (TLR) 7 leading to the production of cytokines and chemokines. It augments both innate and adaptive immune responses by enhancing cytotoxic T-cell-mediated immune responses and by the recruitment of plasmacytoid dendritic cells (CD123+) to the skin.1024. and 1025. An inflammatory cell infiltrate develops in 3–5 days. 1026 There is a reduced expression of bcl-2 and an increased apoptotic index.1027. and 1028. Resiquimod, which activates through both TLR7 and TLR8, is more potent than imiquimod in inducing cytokines in vitro. 446 Its use, so far, has been confined to actinic keratoses.
Radiation therapy alone or in combination with surgery is used to treat basal cell carcinoma with perineural invasion. 1029 There are no therapeutic trials that give any guidance for the management of basal cell carcinoma with perineural invasion. The author intends to follow up 100 consecutive cases with perineural invasion to assess their outcome and relate it to treatment, site of the lesion, and the initial surgical clearance. Very few cases received radiotherapy in the author’s cases. Seven years have now elapsed since these cases were accessioned. Radiotherapy has also been used for tumors of the lip, 1030 and for giant lesions. 1031 Palliative therapy with the epidermal growth factor receptor antagonist cetuximab has also been used for a giant lesion. 1032 Interferon alfa-2b has been used as monotherapy. 1033
In patients who are immunosuppressed, withdrawal of this therapy has resulted in regression of basal cell carcinomas. 1034 Reduction in immunotherapy can also be used in patients with multiple or aggressive tumors.
The reporting of basal cell carcinomas has now become more ‘sophisticated’ with various bodies producing minimum datasets. They were preceded by publications stressing the importance of the histological pattern.1036. and 1037. The minimum dataset produced by the Royal College of Pathologists requires, in essence, the histological type (growth pattern), type of differentiation (presence of a squamous component), presence of perineural invasion, and the distance to the nearest peripheral margin and to the deep margin. 1038 Minimum datasets requiring clearances are controversial, particularly when there is no worldwide consensus on how to respond to the information.
There is considerable variability in the morphology of basal cell carcinomas, and as a consequence a number of histopathological subtypes have been defined. Certain features are shared by more than one of these subtypes, and these aspects will be considered first.
Basal cell carcinomas are composed of islands or nests of basaloid cells, with palisading of the cells at the periphery and a haphazard arrangement of those cells in the centers of the islands. The tumor cells have a hyperchromatic nucleus with relatively little, poorly defined cytoplasm. The intercellular bridges are invisible on routine light microscopy. There are numerous mitotic figures, sometimes atypical, 1039 and a correspondingly high number of apoptotic tumor cells. 1040 This high rate of cell death accounts for the paradoxically slow growth of basal cell carcinomas which possess numerous mitoses. 1041
The vast majority of cases show some attachment to the undersurface of the epidermis. Ulceration is not infrequent in larger lesions. Lesions of long standing and aggressive tumors usually extend into the lower dermis. Deep extension occurs either diffusely or within the paths of the cutaneous adnexae. 1042 Involvement of the subcutis or of the underlying cartilage in lesions of the nose and ear is quite uncommon. 1043 Perineural invasion is present in nearly 1% of cases, although the incidence is higher in aggressive variants.1044.1045. and 1046. Perineural invasion was present in 2.74% of patients in one series of patients treated with Mohs surgery. 1047 It was most often seen in infiltrating, morpheic, and basosquamous types, particularly on the face. 1047
Islands of tumor cells are surrounded by a stroma, which is newly formed and different from the adjacent dermis. Clefting at the stromal–tumor interface is common. 1048 This stroma contains variable amounts of acid mucopolysaccharides. Laminin and types IV, V, and VII collagen are present in the basement membrane, which separates the tumor cells from the stroma.1049. and 1050. However, there is decreased expression of some other basement membrane components, which may facilitate their ability to invade (see above).1051. and 1052. The expression of matrix metalloproteinases may also enhance this ability to invade. 1053 Aggressive basal cell carcinomas show discontinuous staining for laminin and type IV collagen, but a marked stromal myofibroblastic response with an increase in stromal fibronectin.1052. and 1054. They are also more likely to express p53 protein. 1055 Angiogenesis is also present in the stroma. 1056 Amyloid, which is formed by the tumor cells, is present in the stroma in up to 50% of cases.1057.1058. and 1059. It is less common in aggressive variants. 1058 Amyloid may lead to apparent lack of sensitivity to radiotherapy. 1060 The adjacent dermis shows solar elastosis in over 90% of cases, although its degree is sometimes mild. 1061 The overlying epidermis may show the changes of a solar keratosis, although this is only rarely the precursor of a basal cell carcinoma. 1062
A variable inflammatory cell infiltrate is usually present, although there is a paucity of cells in some recurrences. The presence of plasma cells in the infiltrate correlates with ulceration. The infiltrate is usually composed mainly of T cells, the majority of which are CD4+.1063.1064. and 1065. Natural killer cells, mast cells, 1066 and Langerhans cells are also present.1063.1067.1068. and 1069. Cell-mediated immunity appears to play a role in the focal regression seen in up to 20% of tumors.1064. and 1070. The expression of interleukin-2 receptor is increased in regressing lesions. 1064 Active regression is characterized by the presence of a lymphocytic infiltrate which surrounds and penetrates tumor nests, with disruption of the normal palisaded outline and the formation of numerous apoptotic tumor cells.1067. and 1070. Both C4+ and CD8+ cells are present in regressing lesions. 1071 Past regression can be recognized by finding areas of eosinophilic new collagen within a tumor, associated with absence of tumor nests, an increase in small blood vessels, loss of appendages, and a variable inflammatory cell infiltrate. 1070 Prominent central regression with the formation of scar tissue is a feature of the so-called ‘field fire’ type of basal cell carcinoma.
Calcification may be present in the center of the keratin cysts that form in several of the histological subtypes. Ossification is an exceedingly rare event.1072.1073.1074. and 1075. Another rare finding is the presence of transepidermal elimination of tumor nests. 1076 Also rare is the development of pseudoepitheliomatous hyperplasia or keratoacanthoma-like changes in the epidermis following irradiation or excision of a basal cell carcinoma: 1077 this is known as pseudorecidivism. 1078
Basal cell carcinomas or closely related changes may overlie a dermatofibroma. 1079 This has been discussed on page 830. Similar basaloid proliferations may overlie a cutaneous myxoma1080 and connective tissue/mesenchymal hamartomas. 1081 Basal cell carcinomas have also been reported in association with seborrheic keratoses, intradermal nevi, 1082 porokeratosis, Darier’s disease, lupus vulgaris, keratoacanthoma, desmoplastic tricholemmoma, 1083 neurofibromas and, as already mentioned, organoid nevi, 1084 and trichoepitheliomas. 829 Combined (intermingled) basal cell carcinoma and malignant melanoma are rare. 1085
Sometimes no tumor can be found in a biopsy specimen despite a strong clinical suspicion that basal cell carcinoma is present. This is particularly so with the (multifocal) superficial basal cell carcinoma where nests can be widely spaced or undergo regression. It is good practice to order, routinely, three levels of all punch and shave biopsies to prevent sampling errors. Further levels may be required. Clues in an initial non-diagnostic slide that suggest that deeper sections may yield basal cell carcinoma include focal basal atypia, stromal or superficial fibrosis, empty dermal spaces, equivocal adnexae, and microcalcifications. 1086
The tumor cells in basal cell carcinoma resemble epidermal basal cells, in their glycoconjugate pattern, their keratin expression,1087. and 1088. and the presence of bcl-2.1089.1090.1091.1092. and 1093. They differ by the absence of CD95 (APO-1/FAS), which is present in epidermal keratinocytes, particularly in sun-exposed skin. 1094 Actin, a microfilament that contributes to cell motility and invasiveness of cancer cells, is often present in the nodular component of mixed nodular and infiltrating types but much less often in pure nodular lesions. 1095 The expression of p53 is related to aggressive variants of basal cell carcinoma. 1096 The great majority of basal cell carcinomas express CD10. 1097 The smaller the number of positive tumor cells, the larger the number of positive stromal cells, in particular in sclerosing variants. 1097 Only stromal cells express CD10 in squamous cell carcinomas.1097. and 1098. Clinically aggressive basal cell carcinomas have low labeling with bcl-2.1099. and 1100. The cells also express cytokeratins, which are found only in follicular epithelium.1087. and 1101. CAM5.2 (CK8, CK18, and CK19) may be useful in the distinction of basal cell carcinoma from sebaceoma. It is usually negative in sebaceoma and squamous cell carcinoma and positive in a proportion of basal cell carcinomas and trichoepitheliomas. 1102 In one study, all basal cell carcinomas expressed CK5 and CK17, more than half expressed CK7 and most were negative for CK14. 1103 Basal cell carcinomas express p63, but not usually as strongly as squamous cell carcinomas. 1104 Tumor cells stain with the murine monoclonal antibody VM-1; 1105 they usually do not stain for involucrin, epithelial membrane antigen, or CD44, as occurs in squamous cell carcinomas,1106.1107.1108.1109. and 1110. although CD44 has been found in infiltrative tumor strands. 1111 However, they do stain diffusely for Ber-EP4, unlike squamous cell carcinomas, which are always negative;1102.1112.1113. and 1114. basaloid carcinoma of the anus is also negative. 777 D2-40, a new marker of lymphatic endothelium, is expressed in 65% of basal cell carcinomas, and also in squamous cell carcinomas. 1115 Basal cell carcinomas are negative for epithelial membrane antigen (EMA), except focally in squamous and keratotic areas. 1116 A band-like peritumorous reaction with peanut agglutinin has been reported in most basal cell carcinomas, 1117 but not in trichoepitheliomas. 1118 Other features that can be used to distinguish these two tumors are the staining pattern for bcl-2 (expressed in virtually all cells in most basal cell carcinomas, but only weakly in some aggressive variants, and only in the basal layer of trichoepitheliomas) and CD34 (found in the peritumoral fibroblasts around trichoepitheliomas but not in those around sclerosing basal cell carcinomas).1118.1119. and 1120. Whereas most basal cell carcinomas show epithelial staining with CD10, trichoepitheliomas do not usually have epithelial expression alone, but they usually show stromal expression of CD10. In contrast, stromelysin-3 is expressed by the fibroblastic cells of nearly 70% of morpheic basal cell carcinomas, but not by fibroblasts in desmoplastic trichoepithelioma.1121. and 1122. The expression of class II β-tubulin also differs in these two tumors. 1123 Light microscopy is still the most reliable method of distinguishing these two tumors, although the diffuse staining of basal cell carcinomas with bcl-2 is of some value.1113. and 1124.
Various morphological subtypes have been defined. These include nodular (solid), micronodular, cystic, superficial (superficial multifocal), pigmented, adenoid, infiltrating, sclerosing, keratotic, infundibulocystic, metatypical, basosquamous, and fibroepitheliomatous. 1125 Mixed patterns are quite common (Fig. 31.18). 1036 Carlson and colleagues have presented evidence that a histological continuum exists that moves from low-risk types (superficial and nodular) via less frequent transitional, mixed types toward the high-risk micronodular, morpheic, and infiltrating types. 1126 The host immune response and stromal alterations accompany this progression. Several other rare variants have also been described. It should be remembered that punch and shave biopsy techniques provide approximately 80% accuracy in the diagnosis of the various subtypes of basal cell carcinoma. 1127
Basal cell carcinoma of mixed type with (A) a superficial solid and micronodular component and (B) a deep sclerosing/morpheic area. This lesion had not been treated previously, supporting the view that aggressive lesions may develop de novo, without previous treatment. (H & E)
The nodular variant, also known as the large nest type, accounts for approximately 70% of all cases. It is composed of islands of cells with peripheral palisading and a haphazard arrangement of the more central cells. Retraction spaces sometimes form between the tumor islands and the surrounding stroma. Ulceration may be present in larger lesions. The author uses the term ‘solid’ rather than nodular, because of the frequent use of the term ‘nodular’ to describe a clinical variant of basal cell carcinoma that is not always of this histological subtype.
The micronodular variant resembles the solid type, but the nests are much smaller and the peripheral palisading is not always as well developed (Fig. 31.19). The micronodular type has a much greater propensity for local recurrence than the solid type. 1128 It usually has little or no fibro-inflammatory response, 1126 but clinically it may mimic a sclerosing variant. Sometimes it infiltrates quite widely through the dermis and extends into the subcutis. The micronodular type is often included incorrectly with the infiltrating or nodular (solid) types.
Basal cell carcinoma. The superficial part is of solid type and the deeper nests are of micronodular type. (H & E)
One or more cystic spaces are present toward the center of some or all of the tumor islands. 858 This results from the degeneration of tumor cells centrally, and it may be associated with increased mucin between the tumor cells adjacent to the cyst. Rarely, a cystic basal cell carcinoma can mimic a hidrocystoma on a superficial biopsy. 1129
A three-dimensional reconstruction study has shown that the apparently discrete nests of tumor cells are interconnected, suggesting a unicentric origin. 1130 The author has stubbornly continued to include the word ‘multifocal’ in the title, because of the wide separation of the nests in some lesions. However, the finding that the tumor is monoclonal makes it unlikely that the lesion is other than unifocal in its origin (Fig. 31.20). 1131 The superficial basal cell carcinoma is composed of multiple small islands of basaloid cells attached to the undersurface of the epidermis, and usually confined to the papillary dermis. Acantholysis has been reported in a few cases. 1132 A narrow zone of fibrous stroma may surround the nests. There is usually a patchy band-like lymphocytic infiltrate and an increase in thin-walled vessels. 1133 This pattern accounts for 10–15% of all tumors, and is the usual pattern seen in lesions removed from the shoulder region. 787 The age of patients with this subtype is lower than for other types. 1134 A recent study found a more equal distribution of superficial variants on the face, trunk, and limbs, tending to blur the difference between intermittent and continuous sun exposure as the causative environmental agent. 736
Basal cell carcinoma of (multifocal) superficial type. (H & E)
Melanin pigment is usually formed in solid, micronodular, multifocal superficial, or follicular variants. 1135 Functional melanocytes are scattered through the tumor islands and there are numerous melanophages in the stroma. 1136 There are few melanosomes within the tumor cells. Melanosome complexes form in tumor cells as a consequence of repeated cycles of phagocytosis of melanosome-containing tumor cells that have undergone apoptosis. 1137 Basal cell carcinomas of the usual type are also populated by some melanocytes.1016. and 1138. Other pigmented phenomena in basal cell carcinomas are the colonization of one by a melanoma in situ, the metastasis of a melanoma to one, and the presence of a combined melanoma and basal cell carcinoma.1139.1140. and 1141.
The adenoid variant consists of thin strands of basaloid cells in a reticulate pattern. Stromal mucin is often quite prominent. The adenoid type is quite uncommon in a pure form. It may occur in association with the solid type. The rare trabecular variant has some adenoid features. 1142
This non-sclerosing variant has an infiltrative rather than an expansile pattern of growth. 1143 It accounts for approximately 5% of all tumors, although this figure is higher in some patient groups. 1144 The histological features are distinctive, with elongated strands of basaloid cells, 4–8 cells thick, infiltrating between collagen bundles (Fig. 31.21). 1143 Sometimes, even narrower strands are present, with spiking projections. 1143 There may be a slight increase in fibroblasts, but there is no significant fibrosis. Often there is a solid pattern superficially with the infiltrating nests at the periphery or base of the lesion. Sometimes a focal infiltrative pattern is seen in the re-excision specimen of a biopsy proven solid (nodular) basal cell carcinoma. 1145 These changes are limited to the region of the biopsy scar and appear to represent a scar-induced phenomenon without any sinister connotations. Like the sclerosing variant, it has a clinically indistinct border, but it differs from that variant in its opaque, yellow-white color. 1143 Metallothionein, a presumptive marker of aggressive clinical behavior, is increased in the infiltrative variant. 1146
(A) Basal cell carcinoma of infiltrating type, with (B) elongated nests of basaloid cells infiltrating between the collagen bundles of the dermis. (H & E)
The sclerosing category includes lesions which have also been referred to as fibrosing, scirrhous, desmoplastic, and morpheic.1147. and 1148. The uncommon ‘field fire’ type with central fibrosis resulting from regression should not be included in this category. Up to 5% of all basal cell carcinomas are of the sclerosing (morpheic) type. 1147 The tumor presents as an indurated, pale plaque with a slightly shiny surface and clinically indistinct margins. 1147 There are narrow elongated strands and small islands of tumor cells embedded in a dense fibrous stroma. 1148 If the stroma has dense, eosinophilic areas resembling a keloid, then the term ‘morpheic’ has traditionally been used, although at other times this term has been used interchangeably with ‘sclerosing’. This was apparently the case in a recent series that found morpheic or partly morpheic features in 21.8% of all basal cell carcinomas. 1149 Apparent sclerosing features are not uncommon in superficial shaves of ulcerated basal cell carcinomas but sclerosis is relatively uncommon in deeper portions of these tumors when they are subsequently excised. Truly morpheic lesions do not respond well to most standard therapies, other than surgery. 1149 The term ‘keloidal’ has been applied to basal cell carcinomas with thick sclerotic keloidal collagen bundles in the stroma.1150.1151.1152.1153. and 1154. A selectively enhanced procollagen gene expression has been found in the sclerosing variant. 1155 Also, large defects have been found in the basal lamina that surrounds the tumor nests. 1156 Smooth muscle α-actin and myosin are often present in the stroma.
The keratotic variant is similar to the solid type, with nests and islands of basaloid cells with peripheral palisading. 1157 It differs in the presence of squamous differentiation and keratinization in the centers of the islands. 1158 There is usually very little stroma, and no lobular arrangement or follicular differentiation. 1157
The uncommon infundibulocystic variant, found most often on the face, is often confused with the keratotic type.1159.1160.1161.1162. and 1163. It is small, well circumscribed, and composed of nests of cells arranged in an anastomosing fashion with little stroma (Fig. 31.22). There are numerous small infundibular cyst-like structures containing keratinous material and sometimes melanin. 1159 The stroma may contain amyloid and/or melanin. 1159 Multiple lesions have been reported in a patient with HIV infection. 1164 Hereditary cases (OMIM 604451) appear to form a distinctive genodermatosis that is different from the nevoid basal cell carcinoma syndrome.1165. and 1166. Genetic studies have mapped the defect to chromosome 9q22.3, flanking the PTCH gene. 1165
Basal cell carcinoma of infundibulocystic type. (H & E)
Although the term ‘metatypical’ is sometimes applied to tumors with mixed basaloid and squamous features, it should be reserved for the rare basal cell carcinoma composed of nests and strands of cells maturing into larger and paler cells (Fig. 31.23).1035. and 1158. Peripheral palisading is often lost. The cells express much less keratin 17 and keratin 8 than do the cells in the more usual types of basal cell carcinoma. 1167 Peripheral palisading is less obvious than usual, and the stroma is often prominent. This variant is regarded by some as having metastatic potential. 1168 Metatypical basal cell carcinomas have declined significantly in incidence over the last 20 years. It may have something to do with the declining use of radiotherapy in the primary treatment of basal cell carcinomas. The metatypical areas were often in recurrent lesions.
Basal cell carcinoma of metatypical type. There are larger cells with loss of palisading. (H & E)
Basosquamous carcinoma is a controversial entity which can be defined as a basal cell carcinoma differentiating into a squamous cell carcinoma.1158.1169.1170. and 1171. It is composed of three types of cell: basaloid cells, which are slightly larger, paler and more rounded than the cells of a solid basal cell carcinoma; squamoid cells with copious eosinophilic cytoplasm; and an intermediate cell which resembles that seen in metatypical tumors.1158. and 1171. Accordingly, the basosquamous carcinoma is sometimes confused with metatypical and keratotic basal cell carcinomas. It also needs to be distinguished from basaloid squamous cell carcinoma, which when present in non-genital and non-perianal areas has often arisen in overlying bowenoid atypia. Basaloid squamous cell carcinomas are almost always EMA+/Ber-EP4−. 1172 It is an aggressive infiltrative lesion with metastatic potential.1173. and 1174. Perineural invasion is present in up to 10% of cases. 997 It may show extensive subclinical spread. 1175 This tumor shows some areas of Ber-EP4 positivity, in contrast to squamous cell carcinoma which is always negative. 1114 Furthermore, the distribution of basosquamous carcinomas matches basal cell carcinomas more closely than squamous cell carcinomas. 1116
Fibroepithelioma presents as a soft nodular lesion resembling a fibroma or papilloma, often on the lower part of the back.1176. and 1177. It is composed of thin anastomosing strands of basaloid cells set in a prominent loose stroma (Fig. 31.24). 1178 The cells display a high proliferative index. 1179 Symplastic-type epithelial giant cells were present in one case. 1180 The stroma has no elastic tissue. 1176 Merkel cells are quite prominent, a feature of benign follicular tumors. 1181 Pigmentation is rare. 1182 It has been suggested, and subsequently disputed, that this variant derives its histological pattern from the spread of basal cell carcinoma down eccrine ducts, eventually replacing them with solid strands of tumor.801.1183. and 1184. A significant number of basal cell carcinomas on the sole have a fibroepithelioma-like growth pattern. 1185 A further area of dispute involves the histogenesis of these tumors. While Ackerman and Gottlieb regard them as variants of basal cell (trichoblastic) carcinoma, Bowen and LeBoit contend that they are fenestrated trichoblastomas.1186. and 1187. Androgen receptors were expressed in 10 of 13 cases in one series. 1188 This variant can be diagnosed by its dermoscopy pattern. 1189 A rare cystic variant of fibroepithelioma has been reported. 1190
Fibroepithelioma variant of basal cell carcinoma, with thin cords of cells set in a fibrous stroma. (H & E)
Appendageal differentiation is sometimes present in basal cell carcinomas. 1191 Follicular (pilar) variants have already been mentioned (see above). Matrical and tricholemmal differentiation may also occur:1192.1193.1194.1195. and 1196. basal cell carcinomas that show matrical differentiation require differentiation from matrical carcinomas (see p. 770). The tumor cells express nuclear and membranous β-catenin, and osteopontin.1197. and 1198. β-Catenin, predominantly nuclear, is also seen in other variants, particularly the infiltrative and morpheic types.1199.1200. and 1201. Sebaceous differentiation is sometimes seen in areas of an otherwise typical basal cell carcinoma: such lesions require differentiation from other sebaceous tumors (see p. 774). The vacuolated sebaceous cells express epithelial membrane antigen (EMA), a feature not seen in the usual basal cell carcinoma. 1202 A rare variant with histochemical and ultrastructural features of apocrine differentiation has been reported.1142. and 1203. Tumors with eccrine differentiation also occur, and these shade into lesions best classified with eccrine carcinomas1204. and 1205. (see p. 802).
Other variants include the exceedingly rare granular cell,1206.1207. and 1208. clear cell (Fig. 31.25),1209.1210.1211.1212. and 1213. and ‘signet-ring’ cell (hyaline inclusion) types.1214.1215.1216.1217.1218. and 1219. The clear cell variant appears to begin ultrastructurally as lysosomes, suggesting that it forms part of a spectrum with the granular cell variant. 1220 The granular cells sometimes express CD68. 1208 They usually express Ber-EP4 and MNF116. 1221 Lesions with giant tumor cells and large nuclei have been variously reported as ‘basal cell epithelioma with giant tumor cells’, ‘basal cell carcinoma with monster cells’, and ‘pleomorphic basal cell carcinoma’.1222.1223. and 1224. The giant cells are cycling and they do not appear to represent a senescent change. 1225 There is one report of this variant in which there were stromal giant cells as well. 1226Adamantinoid, 1227schwannoid,1228. and 1229. trabecular, 1142 and neuroendocrine differentiation1230.1231.1232. and 1233. are further variants. It has been suggested that the tumor reported as a basal cell carcinoma with thickened basement membrane was really a tricholemmal carcinoma.1234. and 1235. The rare lesion showing myoepithelial differentiation needs to be distinguished from a carcinosarcoma (see p. 699). 1236 A malignant melanoma metastatic to a basal cell carcinoma simulated the pattern of a basomelanocytic tumor. 1237
Basal cell carcinoma with solid (nodular) and clear cell areas. (H & E)
The tumor cells have a large nucleus and cytoplasm containing a few tonofilaments. There are a small number of desmosomes and some thin processes on the cell surface. 1238 The appearances resemble those of the primary epithelial germ. 1239 Stromal amyloid is sometimes present, and this appears to be formed in the cytoplasm of tumor cells. Myofibroblasts have been found in the stroma. 1240
The 5-year recurrence rate for basal cell carcinomas is approximately 5%, although this varies with the type of treatment.1244.1245.1246. and 1247. The figure rises to 9% with long-term follow-up. 1248 Although one study found almost no difference in the recurrence rate of completely excised tumors and those with tumor at one excision margin, 1249 this is contrary to most reports,1250. and 1251. which have found that the distance to the closest resection margin is an important predictor of recurrence. 1252 In another report the recurrence rate was noted to be 1.2% in cases of adequately excised lesions, 12% if tumor was present within 1 high-power field of a margin, and 33% if tumor was present at the excision margin. 1253 In another study the 5-year recurrence rate was 26% when the margin was involved and 14% in cases with free margins. 1254 Residual tumor is found in only 60% of re-excision specimens following a report of tumor at an excision margin. 1255 Failure to find tumor in all such re-excisions may result from an insufficient amount of tumor surviving to be detected, or because the residual cells spontaneously die or are destroyed by a local inflammatory reaction. Another possibility is that the incompleteness of the initial excision was more apparent than real. The presence of residual tumor is more likely when both lateral and deep margins were initially involved. 1256 Failure to detect tumor in re-excision specimens, combined with a recurrence rate thought to be 30–50%, has resulted in a view that additional resection is not always necessary when margins are positive. 1257 One study found that recurrence was unlikely in incompletely excised basal cell carcinomas of superficial or nodular types, less than 1 cm in diameter, located anywhere except the nose or ears, and with less than 4% marginal involvement. 1258 An accompanying Comment suggested that re-excision should be carried out for incompletely excised lesions. 1259 However, if recurrence does occur, subsequent recurrences are sometimes difficult to control.1260. and 1261. Patients with small primary basal cell carcinomas that appear to have been completely removed after a biopsy procedure are at risk for recurrence without further treatment. 1262
All these studies need to be reconsidered in the context of a more recent study, published in 2005. 1263 It concluded that serial transverse cross-sectioning (bread-loafing) at 4 mm intervals of elliptical excision specimens from facial basal cell carcinomas excised with 2 mm surgical margins is only 44% sensitive in detecting residual tumor at the surgical margin. 1263 Put in another way, the pathology report would indicate negative margins in 56% of tumors in which the surgical margins are actually involved. 1263
Recurrences are more common in lesions on the nose, the nasolabial fold, and the inner canthus but this may in part be related to a difficulty in achieving adequate margins in these sites.1250.1264. and 1265. Periorbital recurrences may be associated with orbitocranial invasion, but fortunately this is quite uncommon. 1266 Infiltrative, micronodular, 1250 and (multifocal) superficial types of basal cell carcinoma are more likely to recur than nodular types.1036.1252.1267.1268. and 1269. Metatypical and sclerosing variants may also be associated with more aggressive behavior.1270.1271. and 1272. Recurrences are also more likely in patients who have multiple skin cancers.1273. and 1274. It is now generally accepted that the majority of recurrent basal cell carcinomas are aggressive from the onset, and that in many cases this can be predicted from the histological appearances of the original tumor.1275. and 1276. Notwithstanding these studies, tumors recurring after previous radiotherapy are usually aggressive and infiltrative. 1277 It is unlikely that all such lesions were of an aggressive type initially. A review of the pertinent literature on this subject in 2004 concluded that both intrinsic biological factors and extrinsic management factors play a role in the development and progression of aggressive basal cell carcinomas. 1278 It should be noted that some studies have shown no correlation between the histological type and the recurrence rate, although a lack of uniformity in the histological classification of basal cell carcinomas makes the comparison of some series almost meaningless.1249. and 1270. Basal cell carcinomas in young adults are not more aggressive than those occurring in older patients, as once thought. 1279
Numerous reports document changes in the cells and stroma of basal cell carcinoma, some of which appear to provide explanations for the aggressiveness or otherwise of particular variants of basal cell carcinoma.1280.1281.1282.1283.1284.1285.1286. and 1287. Tumors showing aneuploidy and hyperexpression of cyclin D1 are more likely to have an unfavorable outcome.999. and 1288. Angiogenesis may be an important step in the acquisition of an aggressive phenotype.1289. and 1290. Acquisition of trisomy 6 by tumor cells may lead to the emergence of metastatic potential. 1291
The usual criteria used for the acceptance of metastatic basal cell carcinoma are a previous or present primary lesion in the skin and a metastatic lesion that has a histological picture similar to that of the primary, and which could not have arisen by direct extension from the primary lesion. 1292 Metastases are rare, occurring in approximately 0.05% of cases.1292.1293. and 1294. This low incidence is probably related to the stromal dependence of basal cell carcinomas, which presupposes that only large tumor emboli with attached stroma are successful in implanting. 1295 Accordingly, it is not surprising that lesions that give origin to metastases are large, ulcerated, and neglected.1296. and 1297. Neglected lesions may also directly infiltrate vital structures such as the brain. 1298 Metatypical features and/or squamous differentiation have also been regarded as important,1168.1173. and 1299. although these changes were present in only 15% of the primary lesions in one review of metastasizing basal cell carcinomas. 1292 Lesions on the scrotum appear to have an increased risk of metastasis. 1300 Immunohistochemical markers for Ki-67, p53, and bcl-2 in the primary lesion did not distinguish between metastatic and non-metastatic tumors, in one small study. 1301 Ber-EP4 positivity of the metastatic deposits helps to distinguish such lesions from metastatic basaloid squamous cell carcinoma. 1302
Metastases occur most commonly in the regional lymph nodes.1303. and 1304. Metastatic basal cell carcinoma was diagnosed by sentinel lymph node biopsy in one case. 1305 It was only carried out because lymphatic invasion was present in the primary cutaneous basal cell carcinoma. 1305 Bones, 1306 lungs, 1307 and liver are less frequent sites of involvement.1297. and 1308. Other organs and the subcutis are rarely affected. 1168 Aspiration metastasis to the lung has been recorded. 1309 The median interval between the diagnosis of the primary lesion and signs of metastasis is approximately 9 years, whereas the interval between the appearance of the metastases and the death of the patient is approximately 1 year. 1292Long survivals have occasionally been reported. 1310 Systemic amyloidosis,1311. and 1312. and a myelophthisic anemia secondary to marrow infiltration1313 have been documented in patients with metastatic basal cell carcinomas.
The nevoid basal cell carcinoma syndrome (basal cell nevus syndrome, Gorlin’s syndrome – OMIM 109400) is a multisystem disorder characterized by multiple basal cell carcinomas with an early age of onset, odontogenic keratocysts, pits on the palms and/or soles, cutaneous cysts, 1314 skeletal and neurological anomalies, and ectopic calcifications.1315.1316.1317.1318. and 1319. Often there is a characteristic facies, with hypertelorism and an enlarged calvaria. 1320 Less common manifestations include lipomas and fibromas of various organs, 1321 fetal rhabdomyoma, 1322 unilateral renal agenesis, 1323 adenoid cystic carcinoma of minor salivary gland, 1324 ovarian cysts, and medulloblastomas.1315. and 1325. Its prevalence is estimated to be 1 in 57 000 to 1 in 164 000.1326. and 1327. The inheritance is autosomal dominant with high gene penetrance and variable expressivity. 1328 The responsible gene, the patched homologue 1 (PTCH1) gene, is at chromosome 9q22.3.1329.1330. and 1331. A mutation in PTCH2 on chromosome 1 has recently been described in this syndrome. 1327 Over a hundred different mutations of PTCH1 have now been reported.1326. and 1332. Total deletion of the gene has also been described. 1333 Sporadic cases of basal cell carcinoma with various mutations in genes of the sonic hedgehog pathway have been found (see above). 947 The PTCH1 gene product is part of a receptor for a protein called sonic hedgehog (SHH), which is involved in embryonic development. When SHH binds to PTCH, it releases smoothened (SMOH), a transmembrane signaling protein. 956 SMOH in turn signals to GSK3β, which phosphorylates Gli-3. SOX9 is another downstream target of the SHH pathway. 957 The PTCH protein is important in skin homeostasis. 1334 The molecular aspects of basal cell carcinoma were reviewed in an excellent article in 2005 by Tilli et al. 956
Only 15% of affected individuals have basal cell carcinomas before puberty, and these may take the form of pigmented macules resembling nevi. 1315 More than 90% of patients with this syndrome have basal cell carcinomas by the age of 40. The development of acrochordon-like basal cell carcinomas may be the first manifestation of the syndrome. 1335 Tumors are usually harmless before puberty and only a small percentage become aggressive in later life.1320. and 1336. The tumors may develop anywhere, including within the palmar pits,1337. and 1338. and on the perineum, 1339 but there is a predilection for sun-exposed areas, such as the face, neck, and upper part of the trunk. 1340 They may vary in number from few to hundreds of lesions. A defective in-vitro cellular response to X-irradiation has been found in the syndrome, 1341 and this may help explain the development of skin tumors in these patients at sites of X-irradiation.1337. and 1342.
The unilateral linear basal cell nevus is an unrelated condition which may be associated with comedones and, rarely, osteoma cutis. 1343 Mutations in the PTCH and SMO genes are not involved in this condition, confirming that it is a separate entity (see p. 764).1344. and 1345.
Multiple basal cell carcinomas can occur in a familial setting without other manifestations of a systemic syndrome. In one such family, segmental distribution of the tumors occurred representing mosaicism. 1346 Tissue and tumor mosaicism of the myotonin protein kinase gene located at 19q13.3 was found in a patient with multiple basal cell carcinomas associated with myotonic dystrophy. 1347 This association with myotonic dystrophy had been reported previously. 1347 Multiple hereditary infundibulocystic basal cell carcinomas have also been reported. Genetic studies have mapped the defect to chromosome 9q22.3, flanking the PTCH gene. 1165 Cases with overlap features between these various types of multiple basal cell carcinoma syndromes have been described. 1348
The whole spectrum of histological variants of basal cell carcinoma is found in the nevoid basal cell carcinoma syndrome. 1350 Calcification, keratinizing cysts, pigmentation, and osteoid tissue have all been said to occur more frequently in basal cell carcinomas occurring in the syndrome than in sporadic cases, although some studies have failed to confirm this. 1350
The cutaneous cysts usually take the form of epidermal cysts. 1314 Occasionally they may have a festooned lining of squamous cells that form keratin without the presence of a granular layer, thus resembling the keratocysts of the jaws. 1351
Squamous cell carcinoma is the second most common form of skin cancer in white people. 1355 Its incidence in an Australian study was 166 cases per 100 000 of the population, the highest in the world; 1356 in Scotland it is 34.7 cases per 100 000. 1357 Its incidence has been increasing in many countries1358 but recent evidence suggests that the rate of increase may now be declining. As it is rare for a cancer registry to record data on non-melanoma skin cancers, it is difficult to obtain accurate and comparable data on the true incidence of these cancers. 1359 However, it is estimated that 400 000–600 000 cases of squamous cell carcinoma of the skin occur worldwide each year. 1360 There is a predisposition for it to arise in the sun-damaged skin of fair-skinned people who tan poorly.1356.1361. and 1362. It is relatively uncommon in black people, in whom the tumors often arise in association with scarring processes. 1363 It is often associated with increased morbidity and mortality in black people. Skin cancer in skin of color was reviewed in 2006. 1364
Most squamous cell carcinomas arise in areas of direct exposure to the sun, such as the forehead, face, neck, and dorsum of the hands.1365.1366.1367.1368. and 1369. The ears, scalp, and vermilion part of the lower lip are also involved, particularly in males. 1365 Lesions on the scalp in organ transplant recipients are a high-risk group. 1370 Non-exposed areas such as the buttocks, genitalia,1371. and 1372. and subungual regions1373.1374.1375.1376.1377.1378.1379. and 1380. are occasionally affected. Lesions on the lower limbs are more frequently seen in elderly women.1381. and 1382. Penile cancer is uncommon in the Western world. Most cases are squamous cell carcinomas of which 5–16% are of verrucous type. 1383 Delayed presentation of squamous cell carcinoma may result in autodestruction of the penis. 1384 Uncommonly, squamous cell carcinomas may develop at sites of chronic ulceration, 1385 trauma, burns, frostbite, 1386 vaccination scars,1387. and 1388. tattoos, 1389 a pyoderma gangrenosum scar, 1390 skin grafts, 1391 fistula tracts, 1392 a dilated pore, 1393 pilonidal sinuses of long standing,1394. and 1395. an epidermal cyst, 1396 hidradenitis suppurativa, and acne conglobata.1397. and 1398. The term ‘Marjolin’s ulcer’ has been used for cancers arising in sites of chronic injury or irritation such as scars, ulcers, and sinuses,1399.1400.1401. and 1402. although Marjolin did not describe the condition with which he is eponymously credited. 1403 Tumors arising in these circumstances are sometimes aggressive, and local recurrences are common. 1400 Metastasis also occurs. 1404
There are isolated reports of the occurrence of squamous cell carcinomas in various conditions such as dystrophic epidermolysis bullosa,1405. and 1406. epidermolysis bullosa acquisita, 1407 Hailey–Hailey disease, 1408 porokeratosis,1409. and 1410. discoid lupus erythematosus, 1411 lichen planus,1412.1413. and 1414. a psoriatic nail bed, 1415 erythema ab igne, leprosy, 1416 lupus vulgaris,1417. and 1418. Klippel–Treunaunay syndrome, 1419 lichen sclerosus et atrophicus,1420. and 1421. balanitis xerotica obliterans, Crohn’s disease, 1422 acrodermatitis chronica atrophicans, chronic lymphedema, 1423 benign lymphoepithelial lesion, 1424 organoid and epidermal nevi,25.1425.1426. and 1427. granuloma inguinale, lymphogranuloma venereum, poikiloderma, 1428 mycosis fungoides, 1429 epidermodysplasia verruciformis, 1430 acrokeratosis verruciformis, non-bullous congenital ichthyosiform erythroderma, 1431 and the Jadassohn phenomenon. They have also developed rapidly in patients with psoriasis or rheumatoid arthritis treated with etanercept, a tumor necrosis factor-α antagonist.1432. and 1433. Squamous cell carcinomas are also increased in frequency among patients with xeroderma pigmentosum, vitiligo, and albinism.1434.1435.1436. and 1437. Several cases have been associated with hypercalcemia.1438.1439.1440. and 1441. Skin cancers arising in patients with non-Hodgkin’s lymphoma are aggressive (high-risk) lesions.1442. and 1443. The colocalization of squamous cell carcinoma and follicular lymphoma has been reported. 1444
Squamous cell carcinomas are found predominantly in older people; 1445 they are rare in adolescence and childhood. 1446 Clinically, they present as shallow ulcers, often with a keratinous crust and elevated, indurated surrounds. The adjacent skin usually shows features of actinic damage. The acantholytic variant is usually a nodular tumor on the head or neck, and is almost invariably misdiagnosed clinically as a basal cell carcinoma. It tends to be more aggressive than the conventional squamous cell carcinoma. 1447 Pigmented variants are rare. 1448 The metastatic potential of squamous cell carcinoma will be considered after discussion of the histopathology.
Patients whose immune status is deficient,1449. and 1450. particularly organ transplant recipients,1451.1452.1453.1454.1455.1456.1457.1458. and 1459. are also predisposed to develop these tumors. It has been estimated that organ transplant recipients have a risk of developing squamous cell carcinoma of the skin which is 18–82 times that of the general population.1460. and 1461. The incidence of carcinoma of the lip is also increased in these patients. 1462 Squamous cell carcinoma is the predominant type of skin cancer in renal transplant recipients with an SCC/BCC ratio of up to 3:1, a reversal of the usual ratio in the community.940. and 941. They also develop warts with varying dysplasia, and verrucous keratoses with a putative viral contribution. 926 In a significant number of these tumors HPV-5 or HPV-8 is found, suggesting a role for the virus in the etiology of the skin cancers that result. 926 The E6/E7 oncogenes of these epidermodysplasia verruciformis (EV) strains of HPV appear to be involved in this carcinogenesis. 1463 In genital lesions HPV-16 and/or HPV-18 have been detected.1464. and 1465. HPV-16 appears to be a risk factor for squamous cell carcinoma of the head and neck. 1466 Human papillomavirus has been specifically excluded in some series of post-transplant skin cancers;1467. and 1468. however, the frequency and spectrum of HPV types detected depend on the HPV detection system used, indicating a need for standardization of techniques. 1469 There may be a synergistic interaction of HPV and HIV in the carcinogenic process leading to the development of some penile carcinomas. 1470 It appears that background levels of p53 mutations may be increased in the normal skin of post-transplant and HIV patients and this may contribute to the increased incidence of skin cancers in these patients.1471.1472.1473.1474. and 1475. It should be remembered that the immunohistochemical overexpression of p53 does not necessarily reflect the degree of p53 gene mutations as short gene deletions will not be reflected in increased p53 immunoreactivity. 1476 There is also an increased telomere length in these patients, suggesting that telomere maintenance mechanisms should be further evaluated in organ transplant recipients. 1477 The tumors often infiltrate widely, indicating their aggressive nature. They have a diminished cellular immune response in the stroma.1478. and 1479. Low-dose retinoids produce a reduction in the number of skin cancers in these post-transplant patients.1480.1481. and 1482. Its effects are short-lived in the treatment and prevention of premalignant lesions. Actinic keratoses recur soon after cessation of the drug. 1483 Most of the fatal cases have been reported from Australia, suggesting that sunlight, which has a profound effect on the cutaneous immune system, 380 plays a role in the formation of these aggressive lesions.1453. and 1484. Transplant recipients are often poorly compliant with the use of sunscreens both before and after transplantation.1485. and 1486. Exposure to sunlight before the age of 30 appears to be a risk factor for the development of skin cancers in renal transplant recipients, 1487 although not necessarily for aggressive ones. Other factors, such as the nature of the underlying disease that led to the need for transplantation, may play a role in predisposing to the development of skin cancer. Patients with diabetes mellitus appear to have a lower incidence of skin cancer than those who had polycystic kidney disease and cholestatic liver disease. 641 Information is now being published which indicates that recipients of cardiothoracic transplants have a greater risk of developing aggressive cutaneous malignancies than recipients of renal transplants. 1488 Cardiac transplant patients generally receive quantitatively more immunosuppression than their renal counterparts and this is the presumed explanation for the significant incidence of cutaneous malignancies in this group.1488.1489. and 1490. In a recent study the risk of squamous cell carcinoma but not of basal cell carcinoma in heart transplant recipients was related to the level of global immunosuppression rather than to one specific drug. 1491 Cessation of immunosuppressants appears to result in deceleration of cutaneous carcinogenesis. 1492 Reduction of immunosuppression is considered a reasonable adjuvant strategy in solid organ recipients who have substantial morbidity and mortality risk from skin cancer.1493.1494. and 1495. An update on the pathogenesis of skin cancer in renal transplant recipients was published in 2008. 940
Ultraviolet-B radiation appears to be the most important etiological factor; ultraviolet-A plays a minor role.537.1496.1497.1498.1499.1500. and 1501. Intentional tanning was considered to be a contributing factor to the increase in tumors on covered sites in a Swedish population. 1502 UVB phototherapy appears to produce no significant risk for the subsequent development of skin cancer, 1503 but PUVA therapy for genital psoriasis has been followed by an increase in genital skin cancers. 1504 Ultraviolet radiation is known to damage the DNA of epidermal cells, but there appear to be other complex mechanisms involved in UV-induced carcinogenesis. P53 is a tumor suppressor gene, the mutation of which has been involved in the genesis of various cancers, including skin cancer.1505.1506. and 1507. Mutant p53 accumulates in the cell nucleus and it can be demonstrated by immunohistochemical methods. Expansion of p53 in the epidermis correlates with sun exposure and sun damage. 1508 Ionizing radiation also induces P53 mutations. 1509 It is increased in actinic (solar) keratoses and squamous cell carcinomas arising on sun-damaged skin.1505.1510. and 1511. A different molecular mechanism may be involved in the development of squamous cell carcinomas in sun-protected areas. 1505 Heat shock protein 105 is overexpressed in squamous cell carcinoma but not in basal cell carcinoma. 1512 Epidermal growth factor receptor (EGFR) is expressed in a minority of tumors. 1513 Squamous cell carcinomas can be produced in various animals with ultraviolet radiation, almost to the exclusion of other types of tumors.1497. and 1514. COX-2 inhibitors are an effective chemopreventive agent in ultraviolet carcinogenesis.1515. and 1516. COX-2 expression and angiogenesis are both early events in the development of squamous cell carcinoma.1517. and 1518. Regular users of non-steroidal anti-inflammatory drugs (NSAIDs) have lower risks of developing squamous cell carcinomas and actinic keratoses than non-users. That is, NSAIDs have a protective effect. 1519
Recent work has shown that the JNK cascade is activated in a majority of human squamous cell carcinomas and represents a potential therapeutic target for human epithelial cancers. 1520 The JNK (mitogen-activated protein kinase 7 (MKK7)/C-JUN-NH(2)-kinase (JNK)/activator protein 1 (AP1)) cascade is activated by the TNF-α receptor (TNFR1). 1520
Less important etiological agents include radiation therapy, 814 arsenic, smoking, 1521 coal tar, and various hydrocarbons. 1522
Human papillomavirus may play a role in immunosuppressed patients (see above);926. and 1464. it has a significant role in genital lesions and, rarely, in squamous cell carcinomas on the finger.1468.1523.1524.1525.1526.1527. and 1528. HPV-associated digital squamous cell carcinoma has a high rate of recurrence but a low rate of metastasis.1529. and 1530. HPV is now being detected in squamous cell carcinomas of immunocompetent patients.1531.1532.1533.1534. and 1535. Both beta and gamma HPV strains appear to be involved. 1536
As already mentioned, etanercept therapy for rheumatoid arthritis has been associated with the development of squamous cell carcinoma, although another study found no risk. 1537 Sorafenib, a multikinase inhibitor, alone, or in combination with tipifarnib, a farnesyl-transferase inhibitor, can result in the development of multiple squamous cell carcinomas of the skin. 1538 Topical tacrolimus and pimecrolimus are probably not of etiological significance. 1539 No etiology is apparent in some cases of squamous cell carcinoma. 1540
The role of gene mutations in the development of this skin cancer is now being studied. It is well known that unique mutations in the P53 tumor suppressor gene are linked to the development of non-melanoma skin cancers (NMSC) in patients who have had exposure to ultraviolet radiation (see above). The CDKN2A gene on chromosome 9p21 plays an active role in the p53 and retinoblastoma (rb) tumor suppressor pathways. 1544 In addition to mutations in the P53 gene, loss of expression of CDKN2A via deletion also plays a role in the pathogenesis of human NMSC.1544. and 1545. These deletions in CDKN2A may arise spontaneously during tumor progression. Inactivation of both genes is also common in carcinomas of the external genital organs. 1546 The genomic loss of 18q may be a significant event in the progression of solar keratosis to squamous cell carcinoma. 1547 Chromosomal instability at 13q14 adjacent to the retinoblastoma tumor suppressor gene (Rb) has been detected in some cases. 1548 Inactivation of the Rb gene can result from interaction with some HPV types.1548. and 1549. Dysregulated DNA mismatch repair is present in some non-melanoma skin cancers, particularly squamous cell carcinomas.1550.1551. and 1552. Allelic imbalance/loss of heterozygosity occurs in the BRCA2 gene region in some cases of cutaneous squamous cell carcinoma. 1360 Aberrations in the fragile histidine triad (FHIT) gene have also been reported in cutaneous squamous cell carcinomas. 1553
It should be kept in mind that, in simple terms, carcinogenesis is a three-step process that consists of initiation, promotion, and progression. 1554
Ackerman has proposed that solar keratoses should be regarded as squamous cell carcinomas de novo and that they should not be regarded as premalignancies or precancers that may convert into squamous cell carcinoma.402. and 1555. Others have agreed with this approach.1556. and 1557.
According to this ‘unitarian’ viewpoint the following conditions are morphological expressions of squamous cell carcinoma:
• solar keratosis and its analogues, arsenical and radiation keratoses
• Bowen’s disease and bowenoid papulosis
• giant condyloma and verrucous carcinoma
• keratoacanthoma
• proliferating tricholemmal cysts.
However, this approach assumes the inevitable progression of solar keratoses to squamous cell carcinomas, which is not proved. It is also likely to alarm patients who have one of these lesions. This approach also has a significant impact on reimbursement schedules in some jurisdictions.
There are no large, randomized studies in which different treatments for this tumor are compared. Nevertheless, multiprofessional guidelines for the management of patients with squamous cell carcinoma were published in 2002. 1558 Many factors, including the site of the lesion, its size, and the experience of the practitioner in performing a particular treatment, influence the management of a particular patient.
Where feasible surgical excision (including Mohs surgery where appropriate) is the treatment of choice. Clinical margins of 4 mm have been recommended.1558. and 1559. This measurement does not correspond to the histological clearance, except in well-delineated tumors. For high-risk lesions, where the margins are often poorly defined, a clinical clearance of 6 mm has been recommended. 1558 It should be remembered that in one study, a correct clinical diagnosis of squamous cell carcinoma was made prior to surgery in only 32% of cases, 1560 so that these guidelines should be followed with some caution, in case the clinical diagnosis is incorrect. Surgery is also the treatment of choice for lesions arising in leg ulcers,1402. and 1404. and for lesions of the nail apparatus. 1379
Mohs surgery is superior to traditional surgery particularly for high-risk and facial lesions. In an Australian study of 1263 cutaneous squamous cell carcinomas treated by Mohs surgery, the 5-year recurrence rate was 2.6% for primary lesions and 5.9% for patients with recurrent lesions. 1561
Radiotherapy has been used for lesions of the ear, lip, and nasal vestibule where cosmetic results are important. There was no cartilage damage in one series involving radiation to tumors of the ear. 1562 If perineural invasion is present, adjuvant radiotherapy may be of benefit but there are no comparative trials of its use and non-use in lesions with perineural invasion. 1563
For locally advanced lesions chemoradiation has been used. 1564 The chemotherapeutic agents in one series were low-dose cisplatin and 5-fluorouracil. 1564
In organ transplant recipients with many tumors, curettage and electrodesiccation can be a safe therapy for appropriately selected low-risk lesions. 1565 Excellent cure rates have been achieved using this technique by experienced operators in treating small squamous cell carcinomas on sun-damaged skin in other (non-transplant) patients. 1558 Cryotherapy can also be used for small lesions, but is not appropriate for recurrent disease. 1558
The usual squamous cell carcinoma consists of nests of squamous epithelial cells which arise from the epidermis and extend into the dermis for a variable distance. The cells have abundant eosinophilic cytoplasm and a large, often vesicular, nucleus. There is variable central keratinization and horn pearl formation, depending on the differentiation of the tumor. Individual cell keratinization is often present. The degree of anaplasia in the tumor nests has been used to grade squamous cell carcinomas. Usually, a rather subjective assessment of differentiation is made using the categories of ‘well’, ‘moderately’, and ‘poorly’ differentiated, rather than Broder’s classic grading of 1–4, where grade 4 applies to the most poorly differentiated lesions (Fig. 31.26). Surprisingly, only 54% of dermatopathologists in one study graded squamous cell carcinomas. 1566
Moderately well differentiated squamous cell carcinoma. (H & E)
Most squamous cell carcinomas arise in solar keratoses or sun-damaged skin.1035.1567. and 1568. The borderline between a thick solar keratosis and a superficial squamous cell carcinoma is somewhat arbitrary (see p. 678). The interobserver concordance for these lesions is lower than for basal cell carcinoma. 1569 Ackerman has proposed that very superficial squamous cell carcinoma ‘should be termed just that’. 1570 Squamous cell carcinomas sometimes arise in Bowen’s disease, and in these cases the cells are usually non-keratinizing; they may show variable tricholemmal or even sebaceous-like differentiation. 595 These tumors should not be confused with the rare clear cell1213.1571.1572. and 1573. and ‘signet-ring’1574.1575. and 1576. variants of squamous cell carcinoma, both of which have cells with pale cytoplasm; in the case of the ‘signet-ring’ variant the nucleus is eccentric. The situation in the vulva is different. VIN is found adjacent to squamous cell carcinomas in up to 80% of cases. Many of these tumors are of the classic keratinizing type. 1577 While warty and basaloid tumors were once regarded as an indication of HPV infection, and keratinizing lesions a feature of non-HPV-related squamous cell carcinomas of the vulva, many exceptions occur. 1578 Immunostaining for p16 is a reliable marker for HPV-positive tumors at this site. 1579 Tumors developing in renal transplant recipients cannot be reliably distinguished from those arising in immunocompetent people, 1580 although in one study there was a decreased density of peritumoral inflammatory cells in transplant recipients. 1581 This was postulated to be the reason for their more aggressive behavior. 1581
Squamous cell carcinomas occasionally infiltrate along nerve sheaths, the adventitia of blood vessels, lymphatics, fascial planes, and embryological fusion planes.1582. and 1583. The presence of perineural lymphocytes is an important clue to the likely presence of perineural invasion in deeper sections. 1584 There is often a mild to moderate chronic inflammatory cell infiltrate at the periphery of the tumor. 1585 A subpopulation of these cells express the TCR-αβ heterodimer. 1586 Eosinophils are occasionally prominent in the infiltrate and sometimes extend into the tumor islands. 1587 Erythrophagocytosis by tumor cells has been reported in one case. 1588 The tumor cells may evoke a stromal desmoplastic response. 1589 Stromal components may be important in regulating the growth of some tumors. 1590
Little mention has been made in the literature of vascular invasion by squamous cell carcinoma. The author has seen approximately 10 cases of this phenomenon involving both venules and small arteries. It may result in a tumor thrombus occluding the vessel. 1591
Immunoperoxidase studies are sometimes helpful if the tumor is poorly differentiated or of spindle-cell type. 1592 The cells are positive for epithelial membrane antigen,1114. and 1593. MNF116, and cytokeratin 5/6.1594. and 1595. Squamous cell carcinomas contain keratins of higher molecular weight than those in basal cell carcinomas. 1596 Involucrin is present in larger keratinized cells. 1106 Tumor cells do not express Ber-EP4 or CD10, in contrast to basal cell carcinomas which do. 1102 Some sarcomatoid squamous cell carcinomas may express CD10. Stains for lysozyme, S100 protein, and desmin are negative. Vimentin may be expressed in poorly differentiated and spindle-cell variants (see below). The expression of Ki-67, MIB-1, and vascular endothelial growth factor is higher in squamous cell carcinoma than in basal cell carcinoma.1597. and 1598. However, the level of expression of Ki-67 and MIB-1 is not a predictor of prognosis. 1599 Positive staining for p63 is a highly specific marker for squamous cell carcinoma. 1600 Minichromosome maintenance 5 protein (MCM5) is expressed in nearly 80% of cells in a squamous cell carcinoma. It is a useful marker to detect cell proliferation in skin sections. 1601 CK20 was expressed in two cases of signet-ring cell carcinoma. 1602
Spindle-cell squamous carcinoma is an uncommon variant that usually arises in sun-damaged or irradiated skin. A spindle-cell morphology is more common in squamous cell carcinomas arising in organ transplant recipients. 1603 Rare cases occur on the vulva. 1604 It may be composed entirely of spindle cells, or have a variable component of more conventional squamous cell carcinoma.1605.1606.1607.1608. and 1609. Basosquamous and spindle-cell differentiation in the same lesion has been reported. 1610 In another case, an infiltrative basal cell carcinoma recurred as a spindle-cell carcinoma.1611. and 1612. The spindle cells have a large vesicular nucleus and scanty eosinophilic cytoplasm, often with indistinct cell borders (Fig. 31.27). There is variable pleomorphism, usually with many mitoses. The presence of squamous differentiation, dyskeratotic cells, and continuity with the epidermis may assist in making the diagnosis. Electron microscopy and immunoperoxidase markers may be necessary in some circumstances to differentiate this variant from malignant melanoma and atypical fibroxanthoma.1613.1614.1615. and 1616. Some spindle-cell squamous carcinomas may co-express cytokeratin and vimentin, suggesting metaplastic change of a squamous cell carcinoma to a neoplasm with mesenchymal characteristics.1617. and 1618. Vimentin is sometimes found in squamous cell carcinomas arising in Marjolin’s ulcers. 1619 As the spindle cells are often negative with CAM5.2, it is necessary to use a pankeratin marker or ‘cocktail’. MNF116 is suitable for this purpose. Another useful marker for this variant is p63. 1620 In one study of 16 cases, 6 cases expressed AE1/AE3, while 11 expressed CK5/6. 1621 In a recent study, p63 was expressed in 10/12 cases, and the cytokeratin marker 34βE12 in all 12 cases. 1622 It appears to be a promising marker for spindle-cell squamous carcinoma. HER2 is not expressed. 1623
Squamous cell carcinoma of spindle-cell type. (H & E)
The adenoid (acantholytic, pseudoglandular) variant, which is found most often on the head and neck, accounts for 2–4% of all squamous cell carcinomas.1609.1624.1625.1626. and 1627. It consists of nests of squamous cells with central acantholysis leading to an impression of gland formation, 1624 although the peripheral cells are cohesive (Fig. 31.28). Acantholysis is sometimes minimal. Mucin has been reported in some, 1626 but these tumors may have been variants of adenosquamous carcinoma (see p. 699). Adenoid variants often arise from an acantholytic solar keratosis. Sometimes this is in the vicinity of the pilosebaceous follicle. 1626
Squamous cell carcinoma of adenoid (acantholytic) type. (A) The cells at the periphery of the tumor nests are still cohesive. (B) There is some variability in size of the acantholytic cells. (H & E)
Pseudovascular squamous cell carcinoma is a rare variant of adenoid squamous cell carcinoma which may be mistaken for an angiosarcoma. 1628 The terms ‘pseudovascular adenoid squamous cell carcinoma’1629 and ‘pseudoangiosarcomatous carcinoma’1630. and 1631. have also been used for this tumor. It presents as an ulcer or crusted nodule on sun-exposed skin. It has also been reported on the vulva. 1631 The tumor usually has an aggressive behavior, with a high mortality rate. 1632 Microscopically, the lesion is composed of pseudovascular structures lined by cords of polygonal or flattened tumor cells (Fig. 31.29). 1629 The cells express cytokeratin (AE1/AE3 and 34βE12), epithelial membrane antigen, and, sometimes, vimentin.1631. and 1633. They are negative for CD31, CD34, and factor VIII-related antigen. 1629
Pseudovascular squamous cell carcinoma. (A) There are cords of cells with intervening vascular spaces. (H & E) (B) The keratin stain confirms the squamous cell nature of the lesion. (C) A small cluster of cells has not stained but the majority of cells are positive. (MNF116 immunoperoxidase stain)
There are several other histological variants of squamous cell carcinoma, a knowledge of which may prevent misdiagnosis. The clear cell and ‘signet-ring’ types have already been mentioned (see above). The cells express keratin markers. 1575 The relationship between clear cell carcinoma of the skin and tricholemmal carcinoma is controversial. The existence of tricholemmal carcinoma as originally described by Headington (see p. 768) has been questioned.1609. and 1634. As initially described, clear cell carcinoma of the skin is said to lack glycogen, but 38/40 cases of squamous cell carcinoma with clear cells described by Dalton and LeBoit in 2008 had glycogen, but few cases had the other criteria proposed by Headington for tricholemmal carcinoma. Thus a quandary remains – are clear cell carcinoma of Kuo, squamous cell carcinoma with clear cells of Dalton and LeBoit, and tricholemmal carcinoma of Headington all variants of the same entity? Clear cell carcinoma of the penis is a distinctive variant of penile carcinoma. 1635
A pigmented variant also occurs.1448. and 1636. Melanin is found in epithelial tumor cells as well as in macrophages and dendritic melanocytes. The dendritic cells express S100 protein and HMB-45.1637.1638.1639.1640.1641. and 1642. This variant must be distinguished from the squamomelanocytic tumor, in which both a squamous cell carcinoma and melanoma component develop (see p. 756).
The inflammatory squamous cell carcinoma has a dense lymphocytic stromal infiltrate surrounding poorly differentiated squamous nests (Fig. 31.30). Focal regression is sometimes present. This variant appears to have a high metastatic potential.
Squamous cell carcinoma of inflammatory type. There is usually a heavy infiltrate throughout the entire tumor. (H & E)
The pseudohyperplastic variant is a non-verruciform lesion that occurs on the penis. 1643 It is associated with lichen sclerosus of the foreskin. It is composed of well-differentiated keratinizing nests of squamous cells with minimal atypia surrounded by a reactive fibrous stroma. 1643 It may mimic pseudoepitheliomatous hyperplasia on a biopsy.
The follicular squamous cell carcinoma is a poorly recognized neoplasm arising from the wall of hair follicles (Fig. 31.31). 1644 Sixteen cases of this variant were found in a series of 7000 cases of squamous cell carcinoma reviewed from Spain. 1644 Tumors develop in the upper part of the hair follicles with or without involvement of the epidermis bordering the infundibulum of the involved follicle. 1644 The infundibulocystic (infundibular) squamous cell carcinoma is the term coined by Kossard et al for a tumor which in its well-differentiated form is probably a keratoacanthoma but which in its less differentiated form has probably been regarded as just a squamous cell carcinoma. 1645 It is closely related in some cases to what has been called follicular squamous cell carcinoma. An infundibulocystic hyperplasia that may progress to keratoacanthoma or squamous cell carcinoma has been seen in hypertrophic lichen planus. 1646
Squamous cell carcinoma of follicular origin. (H & E)
The basaloid squamous cell carcinoma is a distinctive variant of squamous cell carcinoma found in the oropharynx1647 and anogenital region. 1648 In this latter region it has a high association with HPV infection. It is composed of small basaloid cells with a high mitotic rate. 1648 Central comedonecrosis is sometimes present in the tumor cell islands. It has a higher histological grade, deeper extension, and higher mortality rate than conventional tumors. However HPV-16 positivity in basaloid tumors of the head and neck is associated with a better survival than tumors that are HPV negative. 1649
Other rare variants are the infiltrative type (Fig. 31.32) with small nests and strands or single cells infiltrating a dermis which is fibrous and/or mucinous,1650. and 1651. and a desmoplastic variant analogous to morpheic basal cell carcinoma.1652. and 1653. A poorly differentiated squamous cell carcinoma with osteoclastic giant cells has been reported.1654. and 1655. The osteoclast-like cells strongly expressed CD68 but not cytokeratin. 1654 Finally, there have been reports of rhabdoid differentiation in a squamous cell carcinoma.1655. and 1656.
(A) Squamous cell carcinoma of infiltrative type. (B) There are strands and small clusters of cells infiltrating between collagenous bundles. (H & E)
The tumor cells in conventional lesions have tonofilaments and well-developed desmosomes and some interdigitating microvilli. In some studies of spindle-cell lesions, the cells have shown the features of squamous epithelium with well-developed desmosomes and tonofilaments.1657. and 1658. In other cases there have been cells resembling fibroblasts, as well as cells exhibiting both mesenchymal and epithelial features. 1659 This mixed pattern of differentiation may result from cell fusion rather than metaplasia. 1659
Recurrences are more likely in those tumors with aggressive histological features such as deep invasion, 1660 poor differentiation, perineural invasion, and acantholytic features.1272.1661.1662.1663. and 1664. Aneuploidy does not appear to correlate with the risk of metastasis. 1665 Narrow surgical margins also contribute to local recurrence. 1666 Squamous cell carcinomas developing in patients who are immunosuppressed or who have an underlying malignant lymphoma or leukemia are often more aggressive.1667.1668. and 1669. The majority of squamous cell carcinomas are only locally aggressive and are cured by several different methods of treatment. The recurrence rate is approximately double that for basal cell carcinomas. 1670
The risk of metastasis varies with the clinical setting in which the lesion arises. 1671 The lowest risk is for tumors arising in sun-damaged skin, and less than 2 cm in diameter.1672. and 1673. The usually quoted figure of 0.5%1672has been challenged as being too low on the basis of several hospital series, which might be expected to be weighted in favor of more aggressive and deeply invasive lesions. 1497 In this regard it appears that vertical tumor thickness is a prognostic variable, just as it is for melanomas,1660. and 1674. although further studies are required to ascertain the critical tumor thickness required for metastasis. Tumors >4–5 mm thick are regarded as high-risk lesions. 1675 Acantholytic variants arising in sun-damaged skin have a slightly greater risk of metastasis, of the order of 2%, 1671 although in one study it was as high as 19%. 1627 Invasive lesions arising in Bowen’s disease metastasize in 2–5% of cases. 595 They have been regarded as high-risk squamous cell carcinomas. 1676 Poor differentiation, incomplete excision, and an immunosuppressed state are other high-risk features. 1675
For lesions arising in skin not exposed to the sun, the incidence of metastases is approximately 2–3%.1677. and 1678. There is a further increase in this risk for lesions of the lip, although the quoted range (2–16%) is quite wide.1674.1678.1679. and 1680. Tumors of the lip are more likely to metastasize if there is perineural invasion, if the tumor is of high grade with a dispersed pattern, and if the tumor thickness exceeds 2 mm.1674. and 1681. The presence of desmoplasia and an inflammatory response with eosinophils and plasma cells are two further adverse features reported recently. 1682 Metastasis is almost invariable for tumors thicker than 6 mm. 1681 Muscle invasion is not useful in predicting the development of lymph node metastases, as was once thought. Thickness of the primary lesion is also a determinant of metastatic potential in squamous cell carcinoma of the skin, in sites other than the lip. Metastasis does not occur in lesions less than 2 mm thick. Metastasis is unusual in tumors confined to the dermis, in the absence of perineural invasion. 1683 Measuring tumor thickness for all squamous cell carcinomas is advocated by some. 1683 The author usually does so for all tumors of the lip and for cutaneous lesions invading beyond the dermis. In a survey published in 2003, only 8% of dermatopathologists stated that they measured tumor thickness. 1566 This parameter is listed as a requirement in the minimum dataset produced by the Royal College of Pathologists. 1684 Tumor vascularity is not a risk factor for metastasis. 1685 A strong dendritic cell response appears to be favorable. 1686 In a retrospective review of 323 patients with squamous cell carcinoma of the lip, the cause-specific survival at 10 years was 98%. 1687 For squamous cell carcinomas arising in Marjolin’s ulcers the incidence of metastasis is thought to be 10–30%, 1678 whereas for vulval, perineal, and penile tumors it may be as high as 30–80%.1671.1688.1689. and 1690. Carcinomas arising in scars, usually resulting from thermal burns, have been regarded in the past as having a higher metastatic rate than non-scar cancers, but this was not confirmed in a recent study, although the follow-up period was short. 1691
Perineural invasion can be divided into two types: incidental, which is detected on biopsy or excision in a patient who is asymptomatic; and clinical, which accounts for 30–40% of cases, in which the nerve invasion has produced clinical symptoms. 1692 Symptomatic patients can be further categorized into imaging-positive and imaging-negative groups. 1692 Radiotherapy gives good results for symptomatic, imaging-negative cases. 1692 In a study on penile squamous cell carcinomas histological grade and perineural invasion were found to be more important than tumor thickness as predictors of nodal metastasis. 1693 In a series of 70 patients from Australia with squamous cell carcinoma and perineural invasion treated by Mohs surgery with adjunctive radiotherapy in 52.9% of cases, the subsequent 5-year recurrence rate was 8%, but only 25 patients had completed this period of follow-up. 1694
Metastases usually occur in the regional lymph nodes in the first instance. For this reason, sentinel lymph node biopsy has been advocated for some high-risk lesions.1695.1696.1697. and 1698. Uncommonly the lung is involved, and then it is usually a terminal phenomenon associated with metastases to other organs. Cutaneous metastases are exceedingly rare. 1699 In one case they produced a bowenoid (epidermotropic) pattern. 1700 A zosteriform pattern of cutaneous metastasis has also been recorded. 1701 A circulating tumor antigen, thought to be specific for squamous cell carcinoma, has been detected in patients with metastatic disease; 1702 however, this antigen has been reported recently in patients with inflammatory dermatoses. 1703 The possibility of a cutaneous metastasis from a visceral organ (such as the lung) should be kept in mind when a rapidly growing nodule presents in the skin. 1704
It has been suggested that tumor spread may be facilitated by a loss of skin-derived antileukoproteinase (SKALP), an inhibitor of elastase and proteinase 3. This enzyme is found in well-differentiated tumors but is absent in poorly differentiated tumor cells. 1705 Other factors undoubtedly contribute, such as reduced expression of the adherens junction protein vinculin1706 and the surface proteoglycan syndecan-1.1707. and 1708. Up-regulation of desmoglein-2 correlates with the risk of metastasis. 1709 There is increased expression of the matrix metalloproteinases (which degrade extracellular matrix),1053. and 1710. of cathepsin B and D,1711. and 1712. of carcinoembryonic antigen, 1713 and of ornithine decarboxylase. 1714 The expression of p14 in vulvar carcinomas is associated with longer survival, while in patients with HPV-related tumors, the expression of high levels of p53 and low p14 indicated the poorest 5-year disease-specific survival. 1715 Chemokine receptor expression may adversely affect the biological behavior of tumors. 1716 Several cases of burn scar-related squamous cell carcinoma, an aggressive variant, have had mutations in the Fas gene, involved in cell death signaling. 1717 Apoptosis is increased in squamous cell carcinomas. 1718 HPV of the same subtype as seen in the primary lesion can be detected in metastatic lesions. 1719
A recent study of the mortality risk from squamous cell carcinoma found that lesion size ≥4 cm, histological evidence of perineural invasion, and deep invasion beyond subcutaneous structures were the features most significantly associated with disease-specific mortality in cutaneous lesions. 1720 The 3-year disease-free survival was 100% for patients with no risk factors versus 70% for patients with at least one of these three risk factors. 1720 Mortality for squamous cell carcinoma appears to be increasing.
A not uncommon problem is the finding of a predominantly cystic squamous cell carcinoma in the soft tissues of the neck. Often, no primary tumor can be found although there may be a history of past skin cancers. One study has shown that, in the majority of cases, the primary lesion will be in the faucial or lingual tonsillar crypt epithelium. 1721
Verrucous carcinoma was first described in the mouth by Lauren V. Ackerman in 1948. 1722 He regarded it as a distinctive variant of squamous cell carcinoma. In addition to the oral cavity,1723.1724. and 1725. it may also involve the larynx, esophagus, and skin.1726.1727. and 1728. It is a slow-growing, often large, warty tumor which invades contiguous structures but rarely metastasizes. 1729 Cutaneous lesions are usually in the genitocrural area1730.1731.1732.1733. and 1734. or on the plantar surface of the foot (epithelioma cuniculatum), although exceptionally it can arise in any part of the skin surface.1735.1736.1737.1738.1739.1740.1741. and 1742. A verrucous carcinoma of the lip has been reported in a young pregnant woman. 1743 The synchronous development of verrucous carcinoma and cutaneous T-cell lymphoma has also been reported. 1734
Plantar lesions are the most common form of verrucous carcinoma.1744.1745.1746.1747.1748. and 1749. They are usually exophytic, pale lesions, sometimes with draining sinuses, 1750 and are often painful and tender. Similar lesions have rarely been reported on the palm or thumb.1751. and 1752. Some cases in these sites, and also on the penis, are microscopically endophytic and the term epithelioma (carcinoma) cuniculatum is still used for tumors with this growth pattern. 1753 HPV-31 and HPV-33 were isolated from a recurrent lesion of the finger with this growth pattern. 1754 Seven cases of ‘carcinoma cuniculatum’ of the penis were reported recently. 1753 None of the tumors showed metastatic spread. Exophytic verrucous carcinomas also occur on the penis. 1755
Occasionally, explosive growth occurs after a prolonged period of slow progression. The mean duration of lesions at the time of diagnosis is 13–16 years. 1756 The incidence of metastasis is low; fatalities are rare, but recorded. 1757
Various factors have been implicated in the etiology of verrucous carcinomas. The chewing of tobacco or betel may predispose to oral lesions, whereas human papillomavirus has been implicated in the etiology of genitocrural lesions1735 and, rarely, of tumors at other sites. 1758 HPV-6, 11, 16, and 18 have been implicated in various cases.1728.1759.1760.1761. and 1762. However, if the concept of warty carcinoma is adopted (see below) then the incidence of HPV in verrucous carcinomas will considerably decrease. A case of plantar verrucous carcinoma has been reported contiguous with a plantar wart, and this may have been of etiological significance. 1763 A histological pattern of lichen simplex chronicus with a variable verruciform pattern and altered epidermal differentiation has been reported on the vulva as vulvar acanthosis. 1764 The lesions were HPV negative. The authors speculated that this lesion might be a precursor to, or a risk factor for, vulvar carcinoma. 1764 Trauma and chronic irritation have also been implicated in the etiology of verrucous carcinoma. 1765 Verrucous carcinoma needs to be distinguished from verrucous psoriasis.1766. and 1767.
Surgical excision and Mohs micrographic surgery are the treatments of choice. 1741 Some lesions are voluminous and tumor mass reduction may be indicated before surgical excision. 1768 Other therapies used include imiquimod, 1769 carbon dioxide laser, cryosurgery, radiotherapy, photodynamic therapy, and topical or systemic chemotherapy. 1768 Intra-arterial infusion of methotrexate was used for a large verrucous carcinoma of the lip. 1770 Imaging is sometimes used to assess the extent of the lesion prior to therapy. 1771
Multiple biopsies are often required for the diagnosis of verrucous carcinoma. Lesions are both exophytic, with papillomatosis and a covering of hyperkeratosis and parakeratosis, and endophytic (Fig. 31.33). 1748 The rete pegs have a bulbous appearance and are composed of large well-differentiated squamous epithelial cells with a deceptively benign appearance. 1772 These acanthotic downgrowths sometimes extend into the deep reticular dermis and even the subcutis. 1773 They are blunted projections, in contrast to the uneven, sharply pointed and jagged downgrowths seen in pseudoepitheliomatous hyperplasia. 1772 There is usually only very low mitotic activity and this is confined to the basal layer. 1774 The downgrowths are mostly contained by an intact basement membrane, although frankly invasive features are sometimes present. A more aggressive, cytologically malignant squamous cell carcinoma may sometimes arise in a verrucous carcinoma, either de novo or following X-irradiation of the lesion. 1775 The combination of verrucous and conventional squamous cell carcinoma is called hybrid verrucous-squamous carcinoma. 1776
Verrucous carcinoma. Well-differentiated nests of squamous epithelium extend into the dermis. (H & E)
Other features include the presence of burrows in the surface filled with parakeratotic horn, as well as draining sinuses containing inflammatory and keratinous debris. Keratin-filled cysts may develop within the tumor mass. 1772 Verruco-cystic squamous cell carcinomas appear to be more common in transplant recipients. 1777 The fibrous stroma surrounding the epithelial downgrowths contains ectatic vessels and a variable inflammatory infiltrate, in which eosinophils and neutrophils are sometimes prominent.1744. and 1778. The presence of neutrophils is an important diagnostic clue. Intraepidermal abscesses are often present in lesions of long standing.
A rare entity affecting the glans penis and known as pseudoepitheliomatous, keratotic and micaceous balanitis may present with the histological features of a verrucous carcinoma; in other instances it has features of a hyperplastic dystrophy akin to vulval dystrophy.1779.1780. and 1781. A true verrucous carcinoma may arise within it. 1782 Its etiology is unknown; HPV infection was excluded in one case. 1783
Verrucous carcinoma should be distinguished from the exceedingly rare papillary variant of squamous cell carcinoma, which is a purely exophytic lesion, in contrast to the mixed endophytic and exophytic character of verrucous carcinoma. 1784
The concept of warty (condylomatous) squamous cell carcinoma has been proposed for an exophytic warty tumor of the penis (a similar lesion occurs on the vulva) usually related to HPV-16 infection.1785. and 1786. This tumor is distinguished from verrucous carcinoma on the basis of long and undulating, condylomatous papillae, with prominent fibrovascular cores and a base which is rounded or irregular and jagged. Furthermore, koilocytotic atypia is prominent and diffuse, while it is absent in the ‘pure’ verrucous carcinoma. 1785 Warty carcinoma of the penis appears to have a better prognosis than typical squamous cell carcinoma of the penis. 1787
Proliferating cell nuclear antigen (PCNA) is expressed at the periphery of squamous nests (similar to keratoacanthomas), in contrast to the more widespread distribution in the usual type of squamous cell carcinoma. 1788
Primary adenosquamous carcinoma of the skin is a rare, usually aggressive, tumor with a potential for local recurrence and metastasis.739.1789.1790. and 1791. The head and neck, and penis are favored sites.1653. and 1792. Many cases in the literature have been reported as mucoepidermoid carcinomas,1793. and 1794. but this term is best reserved for morphologically related tumors arising in the salivary gland, lung, and, rarely, the skin.1789.1795. and 1796. Both tumors are probably appendageal in origin, but because of some morphological features in common, and the presence of squamous nests, the two tumors are considered here for convenience. 1797‘Purists’ could also argue for the removal of basal cell carcinoma from this chapter, and the ‘unitarians’ would have squamous cell carcinoma and little else in this chapter.
Adenosquamous carcinomas are usually deeply invasive tumors composed of islands and strands of squamous cell carcinoma admixed with glandular structures containing mucin (Fig. 31.34). 1798 The mucin, which is of epithelial type (sialomucin), is present in the lumen and the cytoplasm of the lining cells. It stains with mucicarmine, Alcian blue at pH 2.5, and the PAS method, and it is digested by sialidase. The cells are positive for epithelial membrane antigen and cytokeratin, whereas those lining the glandular spaces stain for carcinoembryonic antigen.
Adenosquamous carcinoma. (A) There are glandular spaces containing a small amount of mucin. There are no acantholytic cells, distinguishing this lesion from an acantholytic type of squamous cell carcinoma. (H & E) (B) The cells stain with a broad keratin stain. (Keratin immunoperoxidase)
Adenosquamous carcinoma has been confused with adenoid (acantholytic) squamous cell carcinoma, but in the latter there is no mucin and the glandular spaces contain acantholytic squamous cells. 1799 Adenosquamous carcinoma also differs from the case reported as ‘squamous cell carcinoma with mucinous metaplasia’, which was composed of mucin-containing vacuolated cells resembling ‘signet-ring’ cells; there were no gland spaces. 1789
Mucoepidermoid carcinoma is a common tumor of the salivary gland. It may also occur in other organs such as the paranasal sinuses and lung. It is exceedingly rare in the skin.1795.1796.1797.1800.1801.1802. and 1803. It has been reported in the axilla, 1797 on the finger, 1801 on the scalp in a child, 1802 on the eyelid, 1803 and arising within a nevus sebaceus of Jadassohn. 1800
There is evidence that the tumor is adnexal rather than epidermal, but it will be considered here for the reasons stated above, in the introduction to adenosquamous carcinoma.
Treatment is complete surgical excision, using Mohs technique if necessary. Local recurrences may occur after incomplete removal. 1802
The lesion is often well circumscribed; it may be partly cystic. It is not attached to the epidermis. It is composed of three cell types: mucinous, squamous, and clear. The squamous cells are in lobules and the mucigenic and clear cells are admixed. A mucin stain highlights the mucin cells, while all cell types express CK7, pancytokeratin, CEA, and EMA. 1797
Carcinosarcoma of the skin is an exceedingly rare biphasic tumor with approximately 65 cases reported to date.1804.1805.1806.1807.1808.1809. and 1810. Biphasic sarcomatoid carcinoma is a term that is also used for carcinosarcomas, whereas monophasic sarcomatoid carcinoma has been used to describe a neoplasm composed of cytokeratin- and vimentin-positive spindle cells without a distinct carcinomatous component. 1811 Sarcomatoid carcinoma of the penis encompasses cases of biphasic and monophasic origin.1810.1812. and 1813. In one series of 15 cases, inguinal metastases were present in 89% of cases. The distinction between these various terms appears meaningless in the light of their monoclonality and the likely origin of the mesenchymal component in dedifferentiated carcinomatous cells.1814. and 1815.
Carcinosarcomas are primarily tumors of the elderly, with a male sex predominance. They are located on the head in nearly 50% of cases. 1816 There is a significant variation in their size. 1810 In one case the patient had the nevoid basal cell carcinoma syndrome. 1817
This tumor has metastatic potential which is high in the case of penile lesions (see above). 1818 Tumors with adnexal as opposed to epidermal components are high-risk tumors. 1819 Size >4 cm is associated with a worse outcome. 1820
Complete excision of the lesion is the treatment of choice.
For this diagnosis the tumor must contain an intimate admixture of epithelial and mesenchymal elements, both of which are malignant (Fig. 31.35). 1805 In most cases reported, the epithelial component has been a basal, adnexal, or squamous cell carcinoma,1804.1809.1810.1821. and 1822. whereas the mesenchymal component has included fibrosarcoma, 1823 chondrosarcoma, and osteogenic sarcoma, 1824 and sometimes other elements such as leiomyosarcoma, 1815 angiosarcoma, 1825 giant cell tumor of soft parts, 1826 or rhabdomyosarcoma.1809. and 1827. In sarcomatoid basal cell carcinomas there appears to be a predilection for osteosarcomatous differentiation. 1814
(A) Carcinosarcoma. (B) There is a pleomorphic sarcomatous component surrounding basisquamous epithelial nests. (H & E)
Immunoperoxidase stains are of great value in elucidating the components of this tumor. While the cytokeratin marker AE1/AE3 may be negative in the epithelial component, p63 and MNF116 are often expressed in poorly differentiated epithelial cells.1814. and 1828. The mesenchymal component may express vimentin, actin, CD10, CD34, and CD68, depending on its differentiation.1816. and 1823.
Lymphoepithelioma-like carcinoma of the skin is a rare tumor which resembles histologically the nasopharyngeal tumor of the same name.1829.1830.1831. and 1832. Approximately 50 cases have now been reported. It presents clinically as a papulonodular lesion, usually on the face or scalp.1833. and 1834. The shoulder and vulva are rare sites of involvement. 1835 It has arisen in the scar of a previously excised basal cell carcinoma. 1836 Metastasis is uncommon. 1837 Epstein–Barr virus has not been detected in tumor cells.1833.1838.1839. and 1840. Human papillomavirus and simian virus 40 are also absent in this tumor. 1841 Wick’s group believes that this lesion is a morphological manifestation of squamous cell carcinoma. 1842
The tumor may arise in the dermis or subcutis. It is composed of islands of large epithelial cells surrounded by a dense infiltrate of lymphocytes and some plasmacytoid cells (Fig. 31.36). The epithelial cells have a vesicular nucleus and a large nucleolus. There is no evidence of overt squamous differentiation such as the formation of keratin ‘pearls’, intercellular bridges, or dyskeratotic cells. 1842 Adnexal differentiation has been reported in the epithelial nests in several cases.1839. and 1843. Another case showed spindle-cell differentiation. 1844 The overlying epidermis has been reported as normal in many cases, but as dysplastic in a few. 1845
Lymphoepithelioma-like carcinoma. Tumor cells are difficult to distinguish from the surrounding lymphoid cells. (H & E)
The epithelial cells express cytokeratin (Fig. 31.37) and epithelial membrane antigen, but not S100 protein, 1836 whereas the stromal lymphocytes express CD45 (leukocyte common antigen). Some cases have numerous factor XIIIa-positive dendritic cells in the stroma. 1833
Lymphoepithelioma-like carcinoma. This is the same case as Fig. 31.36. There are more epithelial cells than might be expected from the H & E preparation. (Broad keratin immunoperoxidase)
Lymphoepithelioma-like carcinoma must be distinguished from cutaneous lymphadenoma, a variant of trichoblastoma peppered by lymphocytes (see p. 763). It has also been misdiagnosed as lymphocytoma cutis. 1846 Carcinoma with thymus-like differentiation can also mimic this tumor. The presence of Hassall’s corpuscles in the carcinoma with thymus-like differentiation allows a distinction to be made. 1847
A cutaneous horn is a hard, yellowish-brown keratotic excrescence: to earn the designation of ‘horn’ its height conventionally must exceed at least one-half its greatest diameter. 1848 It may be straight or curved, and up to several centimeters in length. 1849 Giant cutaneous horns, grossly similar to the horns seen in animals, are exceedingly rare in humans. 1850 Cutaneous horns are usually solitary. They have a predilection for the face, the ears, and the dorsum of the hands of older individuals. 1851 A rare site is the penis.1852. and 1853. It is a clinical entity which may be associated with many different pathological lesions. Most commonly a horn overlies an actinic keratosis, seborrheic keratosis, inverted follicular keratosis, 1851 tricholemmoma, verruca vulgaris, or squamous cell carcinoma.1848.1854. and 1855. Cutaneous horns of the eyelid often overlie a seborrheic keratosis. 1856 Rarely, there may be an underlying keratoacanthoma, epidermal nevus, lichen simplex chronicus, epidermal cyst, angiokeratoma, or basal cell carcinoma.1857. and 1858. The pathology in the base of the horn is more likely to be premalignant or malignant in lesions with a wide base or a low height-to-base ratio and in lesions occurring on the nose, scalp, forearms and back of the hands. 1855 There is one report of underlying Kaposi’s sarcoma, 1859 and one of psoriasis. 1860
Horns are composed of keratotic material which may be amorphous or lamellated. Any of the lesions mentioned above may be present in the base. Occasionally, there is underlying epidermal hyperplasia without cellular atypia, and in these circumstances a ‘descriptive’ diagnosis of cutaneous horn is appropriate.
The pattern of keratinization is usually epidermal, but a special variant of horn with tricholemmal keratinization has been reported.1861.1862. and 1863. Human papillomaviruses have been suggested as being causally related to these tricholemmal horns, on the basis of intranuclear inclusions demonstrated on electron microscopy. 1864 The tricholemmal horns must be distinguished from the so-called ‘tricholemmomal horn’, 1865 which represents the usual type of horn, with epidermal keratinization, overlying a tricholemmoma. The term ‘onycholemmal horn’ has been used for a tricholemmal horn on the nail groove. 1866 Nail horns showing epidermal keratinization may also occur, particularly on the big toe. 1849 Filiform parakeratotic horns have also been reported. 1867
Dystrophic calcification is a rare secondary phenomenon in keratin horns. 1868
The stucco keratosis (keratoelastoidosis verrucosa) has received little attention in the literature, despite its reported incidence in the elderly of approximately 5%.1869.1870. and 1871. Lesions are usually multiple and distributed symmetrically on the distal parts of the extremities, particularly the legs.1869. and 1870. Individual lesions are small (1–4 mm), grayish-white keratotic papules. The surface scale may be scratched off with the finger to reveal a non-bleeding, slightly scaly surface. The lesions have some morphological resemblance to the hyperkeratotic variant of seborrheic keratosis and to tar keratoses, and their continued separation as a distinct entity is of doubtful validity. Several HPV types were detected in one case reported comparatively recently. 1872
Stucco keratoses have prominent orthokeratosis and papillomatosis. There is some acanthosis, often associated with fusion of the rete ridges. There is little or no inflammatory infiltrate in the underlying dermis, which may show some solar elastotic changes. There is no increase in basaloid cells in the lesions and there are no horn cysts.
A clavus (corn) is a localized callosity which develops a small horny plug, pressure on which is usually painful. It results from pressure or friction, usually from footwear, on the skin overlying a bony prominence.
A clavus is composed of a thick parakeratotic plug set in a cup-shaped depression of the epidermis (Fig. 31.38). There is usually loss of the granular layer beneath the plug and thinning of the epidermis. A few telangiectatic vessels may be present in the upper dermis.
Clavus. A thick parakeratotic plug is set in a cup-shaped depression of the epidermis. (H & E)
A callus is a circumscribed area of hyperkeratosis resulting from chronic friction or pressure. Callosities develop most frequently on the soles, overlying the metatarsal heads, but they may also arise on the hands in manual workers. Other sites may be affected in individuals involved in certain occupations and recreational pursuits. A callus has developed secondary to the friction and pressure on the hand produced by prolonged use of a computer mouse. It has been termed a ‘mousing callus’. 1874Plantar pressures are significantly higher under callused regions of the foot in older people. 1875 Raised pressure may play a role in their development. 1875
The stratum corneum is thickened and compact, resulting in a slight cup-shaped depression of the underlying epidermis. The granular layer may be thickened, in contrast to a clavus (corn) in which it is usually lost (see above). There is usually some parakeratosis overlying the dermal papillae, but much less than in a clavus.
Onychomatrixoma (onychomatricoma), a benign entity described comparatively recently, presents as a yellow discoloration of the nail plate, with transverse overcurvature of the affected nail. 1876 Approximately 30 cases have been reported, including two in children.1877.1878. and 1879. It may rarely present as longitudinal melanonychia or with nail bleeding.1880. and 1881. A giant variant has been reported. 1882 This tumor appears to originate from the nail matrix cells. 1883 A variant has been reported as an onychoblastoma, 1884 but subsequent correspondence has doubted the separate existence of this ‘entity’ from onychomatrixoma. 1885
The tumor is composed of epithelial cell strands originating from the nail matrix and penetrating vertically into the dermis. Some of the strands anastomose. In the central parts of the strands the keratinocytes evolve into parakeratotic cells, orientated along the axis of the strands.1876.1886. and 1887. The keratinocytes express K14. 1888 The fibrous stroma is sharply delineated from the tumor cells. 1889 If the nail plate is not present, the appearances may resemble a fibrokeratoma. They may be distinguished in longitudinal sections by the presence in onychomatrixoma of epithelial-lined invaginations around optical cavities, a stroma organized in two layers, and the absence of a horny corn. 1888
Ko et al have suggested a new nomenclature to characterize the variability in the histological appearance of these tumors. 1879‘Unguioblastoma’ has been suggested for tumors with a predominant epithelial component and ‘unguioblastic fibroma’ for tumors where a cellular stroma is more prominent. The term ‘atypical unguioblastic fibroma’ was used by these authors to describe a third rare neoplasm, in which the cellular stroma showed nuclear pleomorphism and atypia with an increase of mitotic activity. 1879
Onycholemmal carcinoma is a completely different entity described by Alessi et al. 1890 It is a squamous cell carcinoma variant which in place of the usual horn pearls has cystic structures with abrupt transition between squamous cells and homogeneous keratinized material without the formation of keratinohyaline granules. 1890 In a subsequent case report in 2006, the authors infer that the entity may be the same as subungual keratoacanthoma. 1891
The expression of follicular sheath keratins in the normal nail was reported by Perrin in 2007. He found a lack of an inner root sheath-like compartment and of a companion layer. He proposed the terms matrical keratinization and onycholemmal keratinization for the keratinization of the matrix and the nail isthmus, respectively. 1892
Keratoacanthoma was first described in 1889 by Sir Jonathan Hutchinson, as ‘crateriform ulcer of the face’. 1893 Several other terms, including ‘molluscum sebaceum’ and ‘self-healing squamous cell carcinoma’, have been applied since that time; the term ‘keratoacanthoma’ is now universally accepted.1894. and 1895. Notwithstanding this comment, there is a growing trend in some countries to regard keratoacanthoma as squamous cell carcinoma or a variant of it.1896. and 1897. Terms such as ‘squamous cell carcinoma’ and ‘squamous cell carcinoma (keratoacanthoma type)’ are frequently used. 1898 Their use cannot be justified on morphological or biological grounds. ‘Overcall’ can have as serious consequences for the patient as an ‘undercall’.
A keratoacanthoma is most often a solitary, pink or flesh-colored dome-shaped nodule with a central keratin plug developing on the sun-exposed skin of elderly persons. It grows rapidly to a size of 1–2 cm over a period of 2–10 weeks. This is followed by a stationary period of similar duration. It has a tendency to involute spontaneously; this takes 8–50 weeks. 1899 The duration of the lesion is more variable than is generally recognized, and lesions may persist for over a year without involuting. Clinicians frequently discount the possibility of the lesion being a keratoacanthoma in persistent cases. A keratoacanthoma may cause extensive local destruction, particularly if on the nose or eyelids, before regression occurs, and for this reason active treatment is usually advocated. Healed keratoacanthomas may leave a non-ulcerated, crateriform scar, sometimes resembling a ‘moon crater’. 1900 In contrast, non-follicular keratoacanthomas, specifically subungual and palmar lesions, usually persist, possibly reflecting a different cell of origin, not having the same programmed lifespan as the cells in keratoacanthomas arising in hair-bearing skin. 1896
Keratoacanthomas develop most often in the older age groups, particularly in the sixth and seventh decades, and there is a male preponderance. 1901 A keratoacanthoma has been reported on the lower lip of a 14-year-old boy with discoid lupus erythematosus of the lower lip. 1902 A keratoacanthoma developed in a 15-year-old boy in a post-traumatic hypertrophic scar. 1903 It is estimated that one keratoacanthoma is diagnosed for every four squamous cell carcinomas of the skin, although keratoacanthomas are proportionately more frequent in subtropical areas. 1904 Whereas 70% of lesions develop on the face in temperate climates, there is a much greater tendency for lesions to arise on the arms, dorsum of the hands, and the lower extremity in patients in subtropical areas. 1905 In a series of 40 consecutive cases studied by the author (in subtropical Brisbane, Australia), 85% were on the extremities. 1906 Nearly all lesions develop on hair-bearing areas (follicular keratoacanthomas), although there are some exceptions, to be mentioned below. Rare sites of involvement include the eyelids, 1907 conjunctiva, 1908 lip,1909. and 1910. mouth, 1911 glans penis, 1912 vulva,1913. and 1914. perianal region, and male nipple. 1915
Nearly all keratoacanthomas are solitary lesions, less than 2 cm in diameter, arising on skin exposed to the sun. They may arise on areas not exposed to the sun. 1916 However, there are a number of rare clinical variants that are worthy of mention.1894.1917. and 1918.
The author has seen more than 50 cases of a variant of keratoacanthoma in which involution commences at an early stage in its evolution. It is a clinically distinct variant which referring clinicians are able to diagnose with a high degree of accuracy, once they are aware of the entity. The great majority of cases occur on the lower leg. A striking histological feature of this variant has been the presence of a lichenoid infiltrate in every case which always extends well beyond the proliferative component mimicking a lichen planus-like (lichenoid benign) keratosis.
Keratoacanthoma centrifugum marginatum is a rare variant characterized by progressive peripheral growth with coincident central healing.1924.1925.1926. and 1927. Such lesions may be 20 cm or more in diameter. Most cases occur on the leg,1928. and 1929. but the author has seen cases involving the hand, 1930 and one case involving the buttock and perianal region. Rarely, multiple lesions form. 1931 The plaque-like variant reported as keratoacanthoma dyskeratoticum et segregans1932 is probably related. The multinodular variant has multiple nodular tumors, usually at the expanding periphery of the lesion. 1933 In two cases this variant arose in patients with multiple lesions of Ferguson Smith type.1934. and 1935.
Subungual keratoacanthoma may be solitary or it may be associated with multiple keratoacanthomas of the common type: in the latter case more than one subungual lesion may be present. Subungual keratoacanthomas grow rapidly, often fail to regress spontaneously,1936.1937. and 1938. and usually cause pressure erosion of the distal phalanx. 1939 Spontaneous regression of a subungual keratoacanthoma has been reported. 1940 Paradoxically, they are more destructive than squamous cell carcinomas in this site. 1941 They appear to be identical to the subungual keratotic tumors found in incontinentia pigmenti. 1942 Subungual keratoacanthomas can be grouped with lesions derived from mucous membranes as non-follicular keratoacanthomas. 1896 The case reported in 2006 as onycholemmal carcinoma may have been a subungual keratoacanthoma. 1891
There are several distinct clinical types of multiple keratoacanthomas.1943.1944.1945.1946.1947.1948.1949.1950.1951. and 1952. These include the Ferguson Smith type, 1944 the Grzybowski (eruptive) type,1945.1953.1954.1955.1956.1957.1958. and 1959. a mixed group with overlap or unusual features, 1946 a limited type in which the keratoacanthomas are restricted to one side or area of the body,1947.1960. and 1961. and a secondary type in which the lesions develop at sites of trauma, 1917 treatment or an underlying dermatosis. 1962 The author has seen several cases of keratoacanthomas occurring in the setting of prurigo nodularis and excoriations, examples of which were first described by Emery Kocsard and colleagues in 1972 as ‘multiple keratoacanthoma-like neurodermatitis nodularis’. 1963 Multiple persistent keratoacanthomas have also been reported. 1964 Multiple keratoacanthomas may be associated with internal cancer,1965.1966. and 1967. as in Muir–Torre syndrome, in which multiple keratoacanthomas accompany the presence of a primary visceral cancer, particularly of the gastrointestinal tract.1968.1969. and 1970. Multiple keratoacanthomas may also be associated with malignancies of the genitourinary tract, termed keratoacanthoma visceral carcinoma syndrome.1971. and 1972.
The Ferguson Smith type (OMIM 132800) is characterized by the development of a succession of lesions, one or a few at a time, on covered as well as exposed areas of the body, and beginning usually in adolescence.1917.1949. and 1973. These heal, leaving an atrophic and sometimes disfiguring scar. It seems that the variant found in Scottish kindreds is a more aggressive form with an autosomal dominant inheritance and histological appearances which may resemble those found in a squamous cell carcinoma. 1950 The term ‘multiple self-healing squamous carcinomas’ (MSSE) is still applied to this variant.1950. and 1951. The MSSE gene has been mapped to chromosome 9q31. 1974 Potential candidate genes in this region, the xeroderma pigmentosum (XPA) gene and the PTCH gene have been excluded. Sporadic cases have been described. 1971
In the eruptive Grzybowski type there are multiple (often several hundred) papules and nodules of varying size, with an onset in the fifth and sixth decades. 1952 Lesions may develop on the palms and soles and mucous membranes, as well as in the more usual sites. 1975 They may exemplify the Koebner phenomenon. 1975 The lesions may be intensely pruritic. 1952 Human papillomavirus (HPV) of epidermodysplasia verruciformis type has been isolated from the lesions in patients with this variant of keratoacanthoma. 1976 HPV was not detected in another case. 1977 The Witten and Zak type of multiple keratoacanthomas is a rare familial syndrome that combines features of the Grzybowski and Ferguson Smith types. 1978
Although viruses have long been suggested as an etiological agent, there are only isolated examples of their discovery in keratoacanthomas in immunocompetent individuals, 1979 although a more recent study found HPV DNA in 4 of 11 cases tested. 1980 However, DNA sequences of HPV of both genital and cutaneous types have been detected in 20–55% of keratoacanthomas in immunosuppressed patients.1980.1981. and 1982. A keratoacanthoma has been reported in a patient with epidermodysplasia verruciformis in whom HPV-5 and unknown types of HPV were detected elsewhere on the skin. 1983 In animals, chemical carcinogens can produce lesions resembling keratoacanthomas; such tumors develop from the hair follicles. 1984 Exposure to tars, either industrial or therapeutic, will induce lesions in humans. 535 Exposure to excessive sunlight is the most frequently incriminated factor in the etiology of keratoacanthomas. 1985 Other factors include trauma,1917. and 1986. sometimes surgical, 1987 immunosuppressed states (in which the keratoacanthomas are prone to aggressive growth, early recurrence, and even transformation into squamous cell carcinoma), 1451 chronic renal failure1988 (although subsequent correspondence suggested these lesions may have been an acquired perforating dermatosis), 1989 and xeroderma pigmentosum. 1909 They have infrequently followed bites, vaccinations, 1990 the use of imiquimod, 1991 arterial puncture, 1992 burns, 1993 the smoking of ‘Goza’ and ‘Shisha’ in the Middle East, 1910 and PUVA therapy. 1994 Cases have been reported following the use of sorafenib, a new agent being trialed for many solid tumors. 1995 It targets many different kinases. Keratoacanthomas have also developed following the use of infliximab. 1996 They have been known to develop in linear epidermal nevus, 1997 in organoid nevus (nevus sebaceus),1998. and 1999. in prurigo nodularis treated with cryotherapy, 2000 and in other cryotherapy sites, 2001 in a tattoo,1898.2002.2003. and 2004. within an in-situ malignant melanoma, 2005 at the site of treated psoriatic lesions, 2006 and in association with several dermatoses, 2007 particularly hypertrophic lichen planus.1923.2008.2009. and 2010. They have also arisen in lesions of pseudoxanthoma elasticum. 2011
Surgery is said to be the treatment of choice for solitary lesions or when the number of cases is small. 1971 The author has seen many cases treated by shave excision that have regressed completely, even though a few nests reached the deep edge. Curette with or without cautery can also be used. Facial lesions can be treated with Mohs surgery. It is essential that active treatment is used for facial lesions as untreated lesions may cause considerable destruction of the face, particularly lesions on the columella of the nose.
Intralesional injections of methotrexate or 5-fluorouracil can be used to treat keratoacanthomas.1922. and 2012. Intralesional interferon alfa has been tried for large lesions, 1921 and for lesions on the lip. 2013 A few cases have been successfully treated with topical imiquimod,1929. and 2014. even though one case followed its use for the treatment of Bowen’s disease. 1991
Oral retinoids have been used to treat multiple keratoacanthomas and also keratoacanthoma centrifugum marginatum,1931. and 1934. although the latter variant has also been treated with topical 5% fluorouracil, 2015 imiquimod cream, 1929 local radiation, 1930 and surgical excision of the growing peripheral border. Retinoids may not be effective with the Grzybowski type, 1958 although remission has been achieved with cyclophosphamide. 1975
As there is no single consistent histological feature that characterizes a keratoacanthoma, 2016 the diagnosis may cause difficulty for the inexperienced pathologist, particularly if only a snippet biopsy is submitted. However, there should be no difficulty in making a correct diagnosis on excision or shave specimens and in fusiform biopsies, which extend across the central diameter of the lesion for its full width or at least well into its center. A constellation of histological features needs to be assessed. That said, the author regularly diagnoses keratoacanthomas, without equivocation, on 2 mm punch biopsies on the basis of the distinctive pattern of keratinization. A notation to the effect that a superimposed squamous cell carcinoma cannot be excluded is appended to the reports on all such punch biopsies, in persons over the age of 80 years.
Keratoacanthomas are exoendophytic lesions with an invaginating mass of keratinizing, well-differentiated squamous epithelium at the sides and bottom of the lesion. There is a central keratin-filled crater which enlarges with the maturation and evolution of the lesion. Another key feature is the lipping (also known as buttressing) of the edges of the lesion which overlap the central crater, giving it a symmetrical appearance (Fig. 31.39). In some lesions a keratotic plug overlies discrete infundibula, and a central horn-filled crater is not formed. A well-formed crater containing keratin is present in most regressing (involuting) lesions (Fig. 31.40).
Keratoacanthoma. A lesion in the proliferative phase. (H & E)
Keratoacanthoma. This regressing lesion has a large keratin plug. (H & E)
The component cells have a distinctive eosinophilic hue to their cytoplasm and, as they mature, toward the center of the islands of squamous epithelium, they can become quite large (Fig. 31.41). The presence of keratins 14 (K14) and 16 (K16) throughout the tumor suggests differentiation towards the outer root sheath below the opening of the sebaceous duct. 2017 Epithelial atypia and mitoses are not a usual feature. There is a mixed infiltrate of inflammatory cells in the adjacent dermis, and this is sometimes moderately heavy. Eosinophils and neutrophils may be prominent, and these may extend into the epithelial nests to form small microabscesses. There is no stromal desmoplasia except in late involuting lesions. 2018 Extension below the level of the sweat glands is unusual; 2019 if it is present, particularly careful assessment of the other histological features is required. Atypical hyperplasia of the sweat duct epithelium may be present in some cases. 2020
Keratoacanthoma. The keratinocytes are large, particularly toward the center of the tumor cell nests. (H & E)
A poorly recognized variant of keratoacanthoma that lacks an invaginating pattern and lipping (buttressing) is the ‘multiple follicular’ variant. Kossard illustrates one in his recent article on infundibulocystic tumors with the caption ‘broad-based verrucous infundibulocystic hyperplasia seen with en plaque variants of keratoacanthoma’. 1645
Perineural invasion is an incidental and infrequent finding which does not usually affect the prognosis or behavior of the lesion,2021. and 2022. although local recurrence has been reported in two perioral keratoacanthomas with extensive perineural invasion and intravenous growth (Fig. 31.42). 2023 Of the 40 cases reported from the author’s institution, 27 were from the head or neck region. We found no metastasis in the 35 cases for which follow-up information was available. Local recurrence occurred in only one case, and this was not considered to be directly attributable to the presence of perineural invasion. 1906 The author has seen a further 30 cases since that publication (Fig. 31.43). Many were received as consultation cases and involved the face; they had management implications with serious consequences for the patient.
(A) Keratoacanthoma with perineural invasion. (B) There has been no recurrence after 10 years. (C) Another case with perineural involvement. The large size of the nest relative to the nerve is characteristic. (H & E)
(A) Keratoacanthoma with perineural invasion. (B) The involved nerve is shown. This case was diagnosed on biopsy without equivocation by the author. Subsequent excision confirmed the diagnosis. No further treatment was carried out. (H & E)
Intravenous growth has also been recorded in a keratoacanthoma of the scalp. 2024 No recurrence occurred, although wider excision was carried out. The author has now seen eight such cases that have had no untoward consequences (Fig. 31.44).
Keratoacanthoma in a vein. (A) There has been no recurrence. (H & E) (B) The elastic tissue stain confirms this is a vein with intimal thickening. (Verhoeff–van Gieson)
In keratoacanthoma centrifugum marginatum there is progressive involution and fibrosis toward the center of the lesion, although the advancing edge will show the typical overhanging lip with nests of squamous epithelium in the underlying dermis (Fig. 31.45). Collections of mast cells were present in one case. 2025
Keratoacanthoma centrifugum marginatum. There is buttressing of the advancing edge with early regression in the older ‘central’ area. (H & E)
Subungual keratoacanthomas contain more dyskeratotic cells (Fig. 31.46) and fewer neutrophils and eosinophils than the usual keratoacanthoma, and their orientation is more vertical.1936. and 1939. The dyskeratotic cells may show focal calcification (Fig. 31.47). Some of the smaller lesions in the various syndromes of multiple keratoacanthomas may show a dilated follicle or cup-shaped depression filled with keratin and showing only limited proliferation of squamous epithelium in the base.
(A) Subungual keratoacanthoma. (B) There are numerous dyskeratotic cells. (H & E)
Subungual keratoacanthoma. There are calcium salts in the dyskeratotic cells. (Von Kossa stain)
Keratoacanthomas developing in the Muir–Torre syndrome may have an accompanying sebaceous proliferation. 2026 Absence of staining for one of the mismatch repair gene proteins will also be found. 1970
Some authorities believe that the Ferguson Smith type of lesion should be separated from keratoacanthoma. 1951 There is histological support for this view. The Ferguson Smith tumor may have an indefinite edge, some pleomorphism of cells, and the production of only a small amount of keratin. The infiltrate is usually lymphocytic rather than composed of polymorphs. 1951
Keratoacanthoma-like pseudoepitheliomatous hyperplasia has been reported overlying a CD30+ anaplastic large cell lymphoma. 2027 It may also develop in lesions of prurigo nodularis.
Histological features that favor a diagnosis of keratoacanthoma over squamous cell carcinoma include the characteristic low-power architecture with a flask-like configuration and central keratin plug, as well as the pattern of cell keratinization with large central cells that have slightly paler eosinophilic cytoplasm.2016.2019. and 2028. Not every craterifom lesion is a keratoacanthoma: this architecture can occur in squamous cell carcinomas arising over cartilage, such as on the ear and the base of the nose. The infundibulocystic squamous cell carcinoma described by Kossard et al may closely resemble a keratoacanthoma in its early stages. 1645 The pattern of keratinization of the cells resembles a squamous cell carcinoma and not a keratoacanthoma. 1645 In keratoacanthomas, the crater is usually multiloculated and the lips are perforated. 1916 Other features favoring keratoacanthoma include lack of anaplasia, a sharp outline between tumor nests and stroma, 2029 and absence of stromal desmoplasia. The presence of intraepithelial elastic fibers and intracytoplasmic glycogen has also been said to favor the diagnosis of keratoacanthoma.2030. and 2031. Involucrin, a marker for preterminal squamous differentiation, has been demonstrated by immunoperoxidase methods to be present in all but the basal cells of a keratoacanthoma and in a homogeneous pattern, whereas the staining in squamous cell carcinomas was of variable intensity from cell to cell.2032. and 2033. The volume-weighted mean nuclear volume is higher in keratoacanthomas than in squamous cell carcinomas. 2034 Peanut lectin has been demonstrated in the cell membranes of keratinocytes, in a uniform pattern, in keratoacanthomas but not in most squamous cell carcinomas. 2035 The expression of stromelysin-3, collagenase-3, p21, and the oncoprotein C-erbB-2/neu/HER-2 are all higher in squamous cell carcinomas than in keratoacanthomas.2036.2037.2038. and 2039. Most ‘classical’ keratoacanthomas showed normal membranous immunostaining for E-cadherin and the catenins, but in cases purported to be ‘borderline’ the expression of these adhesion molecules resembled that seen in poorly differentiated squamous cell carcinomas. 2040 That is, E-cadherin fails, as a sole marker, in distinguishing difficult cases of keratoacanthoma from squamous cell carcinoma. 2041 A study using markers for both the initiation phase (the cytolytic receptor P2X7) and the end-stage (TUNEL) found them both present in keratoacanthoma. However, strong P2X7 labeling was also present in squamous cell carcinomas, but the location of this label was quite different from that of keratoacanthoma. 2042 The labeling extended more deeply in squamous cell carcinoma. 2042 Both proliferating cell nuclear antigen (PCNA) and MIB-1 (raised against recombinant Ki-67 antigen) are found in the periphery of the squamous nests in keratoacanthoma, in contrast to a more diffuse staining pattern throughout the nests in squamous cell carcinomas, although some overlap occurs.2043.2044.2045. and 2046. Expression of the p53 oncoprotein and the p16 tumor suppressor protein is found in both entities.2015.2044.2047.2048.2049.2050. and 2051. A recent study showed that subungual squamous cell carcinomas had a higher expression of p53 and Ki-67 than subungual keratoacanthomas. 2052 Analysis of loss of heterozygosity reveals only low loss in keratoacanthomas, contrasting with the high loss in squamous cell carcinomas. 1974 Positron emission tomography performed for another purpose showed increased glucose uptake by a keratoacanthoma. 2053
The literature contains many examples of lesions which on clinical, and sometimes even pathological, grounds were regarded as keratoacanthomas, but which on the basis of their subsequent course were reclassified as squamous cell carcinomas.2019. and 2054. There are four possible explanations for this occurrence. 2054 First, the initial diagnosis of keratoacanthoma could have been wrong; this is the usually accepted and most likely explanation. 2028 Second, the initial lesion may have combined a keratoacanthoma and a squamous cell carcinoma. Third, a keratoacanthoma may have transformed into a squamous cell carcinoma, either as a result of therapy or at some point in its evolution (see below).2055. and 2056. Fourth, keratoacanthoma may actually be a variant of squamous cell carcinoma, as proposed recently by Ackerman.2057. and 2058. The author believes with strong conviction that the biological potential and histopathology of keratoacanthomas and squamous cell carcinomas are so different that their continued separation is essential, if mistreatment of keratoacanthomas with perineural and/or venous invasion is to be avoided.
The author has seen many examples in which part of the lesion was an undoubted keratoacanthoma, but in which, usually toward the base or at one edge, there were areas of typical squamous cell carcinoma. Support for the concept of malignant transformation of keratoacanthoma comes from animal experiments, in which ultraviolet irradiation of hairless mice produced some squamous cell carcinomas that appeared to arise in keratoacanthomas. 2059 This transformation also occurs in immunocompromised patients and in the very elderly. 2028 In the year 2000, Sánchez Yus and colleagues reported focal squamous cell carcinoma change in at least a quarter of all cases. 1916 This is considerably higher than the author’s anecdotal experience of about 3%. One explanation might be that keratoacanthoma in subtropical countries is not the same as in temperate lands. However, with the aging of the population, this phenomenon of squamous cell carcinoma arising in a keratoacanthoma is becoming more common. In an unpublished, prospective study of 20 cases of keratoacanthomas in patients over the age of 90 years, the author found that 85% (17 cases) had superimposed squamous cell carcinomas. Several had perineural invasion but the invaded nerves were large, similar to the nerve involvement seen in keratoacanthomas. The tumor in these perineural spaces looked like squamous cell carcinoma but so far, none has recurred to the author’s knowledge, although no formal follow-up study has been done and the lapsed interval is only months. Incidentally, the contrast between the two components is often striking. There are usually no transitional areas. The risk of squamous cell transformation, in keratoacanthomas from patients over the age of 85, is also high.
It should be remembered that keratoacanthomas can recur in up to 8% of cases. 2060 Recurrence is more likely with lesions on the fingers, hands, lips, and pinnae. 2055 Lesions on the nose and eyelids can be very destructive.1907. and 2061.
The published photomicrographs accompanying a report of a giant metastasizing keratoacanthoma show a pattern of keratinization more in keeping with a squamous cell carcinoma than a keratoacanthoma. 2062 Ackerman and colleagues have reported three cases purporting to be keratoacanthomas in which metastasis occurred. 2057 On this basis, they concluded that keratoacanthoma is a squamous cell carcinoma. 2057
The mechanism responsible for the regression of keratoacanthomas is still poorly understood. There is only limited evidence that regression is immunologically mediated. Activated CD4-positive-T lymphocytes expressing the interleukin-2 receptor (IL-2R) are present in the infiltrate. 2063 Langerhans cells are also increased. 2064 As already mentioned lymphocytic regression is a characteristic feature of the abortive variant of keratoacanthoma (see above). Deletion of many of the cells in the usual keratoacanthoma results from their maturation and keratinization, with subsequent extrusion as a keratin plug. Other cells show dyskeratotic changes (filamentous degeneration), and these tonofilament-rich masses are extruded into the stroma where they become incorporated into the dermal collagen. Still other cells undergo cell death at an earlier stage by the process of apoptosis. It is worth recalling that many keratoacanthomas appear to arise from hair follicle epithelium: in the normal follicle the cells have a programmed ability to be deleted by apoptosis, resulting in catagen involution of the follicle. The author is aware that the infundibulum does not participate in catagen, but this does not negate this analogy. The author has seen two cases of keratoacanthoma, one with perineural invasion in which multiple small (infundibular) milia persisted in the scar following involution of the deeper keratoacanthoma. Expression of BCL-2, a proto-oncogene which inhibits apoptosis, is lost in regressing keratoacanthomas. 2065 It has been found that expression of the cyclin-dependent kinase inhibitor p27 is elevated significantly in regressing keratoacanthomas when compared with growing lesions. 2066 This protein induces a G1 block in the cell cycle. Another study has found sialyl-Tn, a tumor-associated carbohydrate expressed on the cell surface, more often in keratoacanthomas than in squamous cell carcinomas. It may be linked to the regression of keratoacanthomas. 2067
References
Epidermal and other nevi
1. Solomon, LM; Esterly, NB, Epidermal and other congenital organoid nevi, Curr Probl Dermatol 6 (1975) 3–56.
2. Rogers, M; McCrossin, I; Commens, C, Epidermal nevi and the epidermal nevus syndrome. A review of 131 cases, J Am Acad Dermatol 20 (1989) 476–488.
3. Kim, SC; Kang, WH, Nevus comedonicus associated with epidermal nevus, J Am Acad Dermatol 21 (1989) 1085–1088.
4. Demerdjieva, Z; Kavaklieva, S; Tsankov, N, Epidermal nevus syndrome and didymosis aplasticosebacea, Pediatr Dermatol 24 (2007) 514–516.
5. Campen, R; Zembowicz, A; Liu, V; Wrone, D, Linear ectodermal cutaneous hamartoma, Int J Dermatol 42 (2003) 376–379.
6. Su, WPD, Histopathologic varieties of epidermal nevus. A study of 160 cases, Am J Dermatopathol 4 (1982) 161–170.
7. Vidaurri-de la Cruz, H; Tamayo-Sánchez, L; Durán-McKinster, C; et al., Epidermal nevus syndromes: clinical findings in 35 patients, Pediatr Dermatol 21 (2004) 432–439.
8. Hayashi, N; Soma, Y, A case of epidermal nevi showing a divided form on the fingers, J Am Acad Dermatol 29 (1993) 281–282.
9. Rogers, M, Epidermal nevi and the epidermal nevus syndromes: a review of 233 cases, Pediatr Dermatol 9 (1992) 342–344.
10. Shah, KN; Honig, PJ; Yan, AC, Bilateral symmetric facial epidermal nevus, J Am Acad Dermatol 56 (2007) S51–S53.
11. Mehregan, AH; Rahbari, H, Benign epithelial tumors of the skin. Part 1: epidermal tumors, Cutis 19 (1977) 43–48.
12. Submoke, S; Piamphongsant, T, Clinico-histopathological study of epidermal naevi, Australas J Dermatol 24 (1983) 130–136.
13. Ersoy-Evans, S; Sahin, S; Mancini, AJ; et al., The acanthosis nigricans form of epidermal nevus, J Am Acad Dermatol 55 (2006) 696–698.
14. Adams, BB; Mutasim, DF, Adult onset verrucous epidermal nevus, J Am Acad Dermatol 41 (1999) 824–826.
15. Sethuraman, G; Khaitan, BK; Tejasvi, T; et al., Verrucous epidermal nevus with unusual features, Pediatr Dermatol 23 (2006) 98–99.
16. Shafi, M; Khatri, ML; Sen, NK, Extensive verrucous epidermal naevus, J Eur Acad Dermatol Venereol 15 (2001) 269–270.
17. Askar, I; Aytekin, S, Linear verrucous epidermal nevus with cutaneous horn, J Eur Acad Dermatol Venereol 17 (2003) 353–355.
18. Basler, RSW; Jacobs, SI; Taylor, WB, Ichthyosis hystrix, Arch Dermatol 114 (1978) 1059–1060.
19. Adam, JE; Richards, R, Ichthyosis hystrix, Arch Dermatol 107 (1973) 278–282.
20. Sobey, GJ; Quarrell, OW; Williams, S; McGrath, HM, Mosaic chromosome 6 trisomy in an epidermal nevus, Pediatr Dermatol 24 (2007) 144–146.
21. Collin, B; Taylor, IB; Wilkie, AOM; Moss, C, Fibroblast growth factor receptor 3 (FGFR3) mutation in a verrucous epidermal naevus associated with mild facial dysmorphism, Br J Dermatol 156 (2007) 1353–1356.
22. Horn, MS; Sausker, WF; Pierson, DL, Basal cell epithelioma arising in a linear epidermal nevus, Arch Dermatol 117 (1981) 247.
23. Dogliotti, M; Frenkel, A, Malignant change in a verrucous nevus, Int J Dermatol 17 (1978) 225–227.
24. Cramer, SF; Mandel, MA; Hauler, R; et al., Squamous cell carcinoma arising in a linear epidermal nevus, Arch Dermatol 117 (1981) 222–224.
25. Levin, A; Amazon, K; Rywlin, AM, A squamous cell carcinoma that developed in an epidermal nevus, Am J Dermatopathol 6 (1984) 51–55.
26. Braunstein, BL; Mackel, SE; Cooper, PH, Keratoacanthoma arising in a linear epidermal nevus, Arch Dermatol 118 (1982) 362–363.
27. Hivnor, CM; Yan, AC; Honig, PJ, Acne arising in an epidermal nevus, Pediatr Dermatol 24 (2007) 534–535.
28. Waltz, KM; Helm, KF; Billingsley, EM, The spectrum of epidermal nevi: a case of verrucous epidermal nevus contiguous with nevus sebaceus, Pediatr Dermatol 16 (1999) 211–213.
29. Saraswat, A; Jain, R; Kaur, I; Kumar, B, Co-localization of epidermal nevi and psoriasis: Are we closer to an explanation? Int J Dermatol 44 (2005) 972–974.
30. Kishida, ÉS; Muniz Silva, MA; da Costa Pereira, F; et al., Epidermal nevus syndrome associated with adnexal tumors, Spitz nevus, and hypophosphatemic vitamin D-resistant rickets, Pediatr Dermatol 22 (2005) 48–54.
31. Cummins, DL; Cohen, BA, Extensive sebaceous/epidermal nevus associated with cardiac arrhythmia, Pediatr Dermatol 24 (2007) 457–458.
32. Solomon, LM; Fretzin, DF; Dewald, RL, The epidermal nevus syndrome, Arch Dermatol 97 (1968) 273–285.
33. Eichler, C; Flowers, FP; Ross, J, Epidermal nevus syndrome: case report and review of clinical manifestations, Pediatr Dermatol 6 (1989) 316–320.
34. Goldberg, LH; Collins, SAB; Siegel, DM, The epidermal nevus syndrome: case report and review, Pediatr Dermatol 4 (1987) 27–33.
35. Mahakrishnan, A, Megalopinna in naevus uniuslateris: a case report, Acta Derm Venereol 61 (1981) 365–367.
36. Happle, R, How many epidermal nevus syndromes exist? A clinicogenetic classification, J Am Acad Dermatol 25 (1991) 550–556.
37. Hodge, JA; Ray, MC; Flynn, KJ, The epidermal nevus syndrome, Int J Dermatol 30 (1991) 91–98.
38. Mostafa, WZ; Satti, MB, Epidermal nevus syndrome: a clinicopathologic study with six-year follow-up, Pediatr Dermatol 8 (1991) 228–230.
39. Ivker, R; Resnick, SD; Skidmore, RA, Hypophosphatemic vitamin D-resistant rickets, precocious puberty, and the epidermal nevus syndrome, Arch Dermatol 133 (1997) 1557–1561.
40. Tay, Y-K; Weston, WL; Ganong, CA; Klingensmith, GJ, Epidermal nevus syndrome: Association with central precocious puberty and woolly hair nevus, J Am Acad Dermatol 35 (1996) 839–842.
41. Chatproedprai, S; Wananukul, S; Prasarnnaem, T; Noppakun, N, Epidermal nevus syndrome, Int J Dermatol 46 (2007) 858–860.
42. Pandhi, D; Reddy, BSN, A rare association of epidermal nevus syndrome and ainhum-like digital constrictions, Pediatr Dermatol 19 (2002) 349–352.
43. Kaur, T; Kumar, B; Chawwla, YK, Epidermal naevi associated with extrahepatic portal venous obstruction, hypoplastic kidney and lymphangiectasia: a new syndromic variant? Br J Dermatol 150 (2004) 618–619.
44. Stosiek, N; Ulmer, R; von den Driesch, P; et al., Chromosomal mosaicism in two patients with epidermal verrucous nevus, J Am Acad Dermatol 30 (1994) 622–625.
45. Inglesias Zamora, ME; Vázquez-Doval, FJ, Epidermal naevi associated with trichilemmal cysts and chromosomal mosaicism, Br J Dermatol 137 (1997) 821–824.
46. Vujevich, JJ; Mancini, AJ, The epidermal nevus syndromes: Multisystem disorders, J Am Acad Dermatol 50 (2004) 957–961.
47. Sugarman, JL, Epidermal nevus syndromes, Semin Cutan Med Surg 26 (2007) 221–230.
48. Dimond, RL; Amon, RB, Epidermal nevus and rhabdomyosarcoma, Arch Dermatol 112 (1976) 1424–1426.
49. Rosenthal, D; Fretzin, DF, Epidermal nevus syndrome: report of association with transitional cell carcinoma of the bladder, Pediatr Dermatol 3 (1986) 455–458.
50. Obasi, OE; Isitor, GN, Extensive congenital bilateral epidermal naevus syndrome – a case report from Nigeria with ultrastructural observations, Clin Exp Dermatol 12 (1987) 132–135.
51. Attia, MK; Abdel-Aziz, AM, Epidermal naevus syndrome, Br J Dermatol 95 (1976) 647–648.
52. Gobello, T; Mazzanti, C; Zambruno, G; et al., New type of epidermal nevus syndrome, Dermatology 201 (2000) 51–53.
53. Rustin, MHA; Bunker, CB; Gilkes, JJH; et al., Polyostotic fibrous dysplasia associated with extensive linear epidermal naevi, Clin Exp Dermatol 14 (1989) 371–375.
54. Samlaska, CP; Levin, SW; James, WD; et al., Proteus syndrome, Arch Dermatol 125 (1989) 1109–1114.
55. Nazzaro, V; Cambiaghi, S; Montagnani, A; et al., Proteus syndrome. Ultrastructural study of linear verrucous and depigmented nevi, J Am Acad Dermatol 25 (1991) 377–383.
56. Child, FJ; Werring, DJ; Du Vivier, AWP, Proteus syndrome: diagnosis in adulthood, Br J Dermatol 139 (1998) 132–136.
57. Cavero, JA; Castro, EG; Junco, L, Proteus syndrome, Int J Dermatol 39 (2000) 707–709.
58. Barona-Mazuera, MdR; Hidalgo-Galván, LR; Orozco-Covarrubias, MdL; et al., Proteus syndrome: new findings in seven patients, Pediatr Dermatol 14 (1997) 1–5.
59. Happle, R, The manifold faces of Proteus syndrome, Arch Dermatol 140 (2004) 1001–1002.
60. Nguyen, P; Turner, JT; Olsen, C; et al., Cutaneous manifestations of Proteus syndrome. Correlations with general clinical severity, Arch Dermatol 140 (2004) 947–953.
61. Farajzadeh, S; Zahedi, MJ; Moghaddam, SD, A new gastrointestinal finding in Proteus syndrome: report of a case of multiple colonic hemangiomas, Int J Dermatol 45 (2006) 135–138.
62. Twede, JV; Turner, JT; Biesecker, LG; Darling, TN, Evolution of skin lesions in Proteus syndrome, J Am Acad Dermatol 52 (2005) 834–838.
63. Loffeld, A; McLennan, NJ; Cole, T; et al., Epidermal naevus in Proteus syndrome showing loss of heterozygosity for an inherited PTEN mutation, Br J Dermatol 154 (2006) 1194–1198.
64. Sapp, JC; Turner, JT; van de Kamp, JM; et al., Newly delineated syndrome of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE syndrome) in seven patients, Am J Med Genet 143A (2007) 2944–2958.
65. Pearson, IC; Harland, CC, Epidermal naevi treated with pulsed erbium: YAG laser, Clin Exp Dermatol 29 (2004) 494–496.
66. Demetree, JW; Lang, PG; St Clair, JT, Unilateral, linear, zosteriform epidermal nevus with acantholytic dyskeratosis, Arch Dermatol 115 (1979) 875–877.
67. Delacretaz, J; Christeler, A, Dyskeratose acantholytique regionale, Dermatologica 163 (1981) 113–116.
68. Starink, TM; Woerdeman, MJ, Unilateral systematized keratosis follicularis. A variant of Darier’s disease or an epidermal naevus (acantholytic dyskeratotic epidermal naevus)? Br J Dermatol 105 (1981) 207–214.
69. van der Wegen-Keijser, MH; Prevoo, RLMH; Bruynzeel, DP, Acantholytic dyskeratotic epidermal naevus in a patient with guttate psoriasis on PUVA therapy, Br J Dermatol 124 (1991) 603–605.
70. Mazereeuw-Hautier, J; Thibaut, I; Bonafé, JL, Acantholytic dyskeratotic epidermal nevus: a rare histopathologic feature, J Cutan Pathol 29 (2002) 52–54.
71. Curth, HO, Unilateral epidermal naevus resembling acanthosis nigricans, Br J Dermatol 95 (1976) 433–436.
72. Vakilzadeh, F; Kolde, G, Relapsing linear acantholytic dermatosis, Br J Dermatol 112 (1985) 349–355.
73. Fletcher, V; Williams, ML; Lane, AT, Histologic changes resembling the verrucous phase of incontinentia pigmenti within epidermal nevi: report of two cases, Pediatr Dermatol 3 (1985) 69–74.
74. Brownstein, MH; Silverstein, L; Lefing, W, Lichenoid epidermal nevus: ‘linear lichen planus’, J Am Acad Dermatol 20 (1989) 913–915.
75. Altman, J; Mehregan, AH, Inflammatory linear verrucose epidermal nevus, Arch Dermatol 104 (1971) 385–389.
76. Morag, C; Metzker, A, Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature, Pediatr Dermatol 3 (1985) 15–18.
77. De Jong, EMGJ; Rulo, HFC; Van De Kerkhof, PCM, Inflammatory linear verrucous epidermal naevus (ILVEN) versus linear psoriasis, Acta Derm Venereol 71 (1991) 343–346.
78. Zvulunov, A; Grunwald, MH; Halvy, S, Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus, Arch Dermatol 133 (1997) 567–568.
79. Kim, JJ; Chang, MW; Shwayder, T, Topical tretinoin and 5-fluorouracil in the treatment of linear verrucous epidermal nevus, J Am Acad Dermatol 43 (2000) 129–132.
80. Alsaleh, QA; Nanda, A; Hassab-El-Naby, HMM; Sakr, MF, Familial inflammatory linear verrucous epidermal nevus (ILVEN), Int J Dermatol 33 (1994) 52–54.
81. Lee, SH; Rogers, M, Inflammatory linear verrucous epidermal naevi: a review of 23 cases, Australas J Dermatol 42 (2001) 252–256.
82. Mazereeuw-Hautier, J; Marty, C; Bonafé, J-L, Familial inflammatory linear verrucous epidermal naevus in a father and daughter, Clin Exp Dermatol 33 (2008) 679–680.
83. Landwehr, AJ; Starink, TM, Inflammatory linear verrucous epidermal naevus. Report of a case with bilateral distribution and nail involvement, Dermatologica 166 (1983) 107–109.
84. Cheesbrough, MJ; Kilby, PE, The inflammatory linear verrucous epidermal naevus – a case report, Clin Exp Dermatol 3 (1978) 293–298.
85. Golitz, LE; Weston, WL, Inflammatory linear verrucous epidermal nevus. Association with epidermal nevus syndrome, Arch Dermatol 115 (1979) 1208–1209.
86. Lee, IW; Ahn, SK; Choi, EH, Inflammatory linear verrucous epidermal naevus arising on a burn scar, Acta Derm Venereol 79 (1999) 164–165.
87. Goujon, C; Pierini, A-M; Thivolet, J, Le psoriasis linéaire; existe-t-il? Ann Dermatol Venereol 108 (1981) 643–650.
88. Sotiriadis, D; Patsatsi, A; Lazaridou, E; et al., Is inflammatory linear verrucous epidermal naevus a form of linear naevoid psoriasis? J Eur Acad Dermatol Venereol 20 (2006) 483–484.
89. Adrian, RM; Baden, HP, Analysis of epidermal fibrous proteins in inflammatory linear verrucous epidermal nevus, Arch Dermatol 116 (1980) 1179–1180.
90. Bernhard, JD; Owen, WR; Steinman, HK; et al., Inflammatory linear verrucous epidermal nevus. Epidermal protein analysis in four patients, Arch Dermatol 120 (1984) 214–215.
91. Bogle, MA; Sobell, JM; Dover, JS, Successful treatment of a widespread inflammatory verrucous epidermal nevus with etanercept, Arch Dermatol 142 (2006) 401–402.
92. Dupre, A; Christol, B, Inflammatory linear verrucose epidermal nevus. A pathologic study, Arch Dermatol 113 (1977) 767–769.
93. Hodge, SJ; Barr, JM; Owen, LG, Inflammatory linear verrucose epidermal nevus, Arch Dermatol 114 (1978) 436–438.
94. Ahrens, EM; Hodge, SJ, Lichen planus arising in an inflammatory linear verrucous epidermal nevus, Int J Dermatol 25 (1986) 527–528.
95. Oskay, T; Kutluay, L, Inflammatory linear verrucous epidermal naevus associated wth ipsilateral undescended testicle, Clin Exp Dermatol 28 (2003) 557–558.
96. Welch, ML; Smith, KJ; Skelton, HG; et al., Immunohistochemical features in inflammatory linear verrucous epidermal nevi suggest a distinctive pattern of clonal dysregulation of growth, J Am Acad Dermatol 29 (1993) 242–248.
97. Grimalt, R; Caputo, R, Posttraumatic nevus comedonicus, J Am Acad Dermatol 28 (1993) 273–274.
98. Inoue, Y; Miyamoto, Y; Ono, T, Two cases of nevus comedonicus: Successful treatment of keratin plugs with a pore strip, J Am Acad Dermatol 43 (2000) 927–929.
99. Lefkowitz, A; Schwartz, RA; Lambert, WC, Nevus comedonicus, Dermatology 199 (1999) 204–207.
100. Guldbakke, KK; Khachemoune, A; Deng, A; Sina, B, Naevus comedonicus: a spectrum of body involvement, Clin Exp Dermatol 32 (2007) 488–492.
101. Beck, MH; Dave, VK, Extensive nevus comedonicus, Arch Dermatol 116 (1980) 1048–1050.
102. Cestari, TF; Rubim, M; Valentini, BC, Nevus comedonicus: case report and brief review of the literature, Pediatr Dermatol 8 (1991) 300–305.
103. Fletcher, CL; Acland, KM; Powles, AV, Unusual giant comedo naevus, Clin Exp Dermatol 24 (1999) 186–188.
104. Abdel-Aal, H; Abdel-Aziz, AHM, Nevus comedonicus. Report of three cases localized on the glans penis, Acta Derm Venereol 55 (1975) 78–80.
105. Ghaninezhad, H; Ehsani, AH; Mansoori, P; Taheri, A, Naevus comedonicus of the scalp, J Eur Acad Dermatol Venereol 20 (2006) 184–185.
106. Cripps, DJ; Bertram, JR, Nevus comedonicus bilateralis et verruciformis, J Cutan Pathol 3 (1976) 273–281.
107. Wood, MG; Thew, MA, Nevus comedonicus. A case with palmar involvement and review of the literature, Arch Dermatol 98 (1968) 111–116.
108. Harper, KE; Spielvogel, RL, Nevus comedonicus of the palm and wrist, J Am Acad Dermatol 12 (1985) 185–188.
109. Paige, TN; Mendelson, CG, Bilateral nevus comedonicus, Arch Dermatol 96 (1967) 172–175.
110. Inamadar, AC; Palit, A; Yelikar, BR, Widespread cribriform plaques in a child, Pediatr Dermatol 21 (2004) 84–86.
111. Engber, PB, The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations, Int J Dermatol 17 (1978) 745–749.
112. Whyte, HJ, Unilateral comedo nevus and cataract, Arch Dermatol 97 (1968) 533–535.
113. Woods, KA; Larcher, VF; Harper, JI, Extensive naevus comedonicus in a child with Alagille syndrome, Clin Exp Dermatol 19 (1994) 163–164.
114. Patrizi, A; Neri, I; Fiorentini, C; Marzaduri, S, Nevus comedonicus syndrome: a new pediatric case, Pediatr Dermatol 15 (1998) 304–306.
115. Alpsoy, E; Durusoy, Ç; Özbilim, G; et al., Nevus comedonicus syndrome: a case associated with multiple basal cell carcinomas and a rudimentary toe, Int J Dermatol 44 (2005) 499–501.
116. Ahn, S-Y; Oh, Y; Bak, H; Ahn, SK, Co-occurrence of nevus comedonicus with accessory breast tissue, Int J Dermatol 47 (2008) 530–531.
117. Lee, HJ; Chun, EY; Kim, YC; Lee, M-G, Nevus comedonicus with hidradenoma papilliferum and syringocystadenoma papilliferum in the female genital area, Int J Dermatol 41 (2002) 933–936.
118. Rodriguez, JM, Nevus comedonicus, Arch Dermatol 111 (1975) 1363–1364.
119. Vasiloudes, PE; Morelli, JG; Weston, WL, Inflammatory nevus comedonicus in children, J Am Acad Dermatol 38 (1998) 834–836.
120. Kirtak, N; Inaloz, HS; Karakok, M; et al., Extensive inflammatory nevus comedonicus involving half of the body, Int J Dermatol 43 (2004) 434–436.
121. Caers, SJ; Van der Geer, S; Beverdam, EGA; et al., Successful treatment of nevus comedonicus with the use of the Erbium Yag laser, J Eur Acad Dermatol Venereol 22 (2008) 375–377.
122. Nabai, H; Mehregan, AH, Nevus comedonicus. A review of the literature and report of twelve cases, Acta Derm Venereol 53 (1973) 71–74.
123. Kurokawa, I; Nakai, Y; Nishimura, K; et al., Cytokeratin and filaggrin expression in nevus comedonicus, J Cutan Pathol 34 (2007) 338–341.
124. Leppard, BJ, Trichilemmal cysts arising in an extensive comedo naevus, Br J Dermatol 96 (1977) 545–548.
125. Marsden, RA; Fleming, K; Dawber, RPR, Comedo naevus of the palm – a sweat duct naevus? Br J Dermatol 101 (1979) 717–722.
126. Abell, E; Read, SI, Porokeratotic eccrine ostial and dermal duct naevus, Br J Dermatol 103 (1980) 435–441.
127. Coskey, RJ; Mehregan, AH, Porokeratotic eccrine duct and hair follicle nevus, J Am Acad Dermatol 6 (1982) 940–943.
128. Aloi, FG; Pippione, M, Porokeratotic eccrine ostial and dermal duct nevus, Arch Dermatol 122 (1986) 892–895.
129. Moreno, A; Pujol, RM; Salvatella, N; et al., Porokeratotic eccrine ostial and dermal duct nevus, J Cutan Pathol 15 (1988) 43–48.
130. Horio, T; Komura, J, Linear unilateral basal cell nevus with comedo-like lesions, Arch Dermatol 114 (1978) 95–97.
131. Blanchard, L; Hodge, SJ; Owen, LG, Linear eccrine nevus with comedones, Arch Dermatol 117 (1981) 357–359.
132. Plewig, G; Christophers, E, Nevoid follicular epidermolytic hyperkeratosis, Arch Dermatol 111 (1975) 223–226.
133. Resnik, KS; Kantor, GR; Howe, NR; Ditre, CM, Dilated pore nevus. A histologic variant of nevus comedonicus, Am J Dermatopathol 15 (1993) 169–171.
134. Price, M; Russell Jones, R, Familial dyskeratotic comedones, Clin Exp Dermatol 10 (1985) 147–153.
135. Hall, JR; Holder, W; Knox, JM; et al., Familial dyskeratotic comedones, J Am Acad Dermatol 17 (1987) 808–814.
136. Carneiro, SJC; Dickson, JE; Knox, JM, Familial dyskeratotic comedones, Arch Dermatol 105 (1972) 249–251.
137. Van Geel, NAC; Kockaert, M; Neumann, HAM, Familial dyskeratotic comedones, Br J Dermatol 140 (1999) 956–959.
138. Leppard, BJ, Familial dyskeratotic comedones, Clin Exp Dermatol 7 (1982) 329–332.
139. Cantú, JM; Gómez-Bustamente, MO; González-Mendoza, A; Sánchez-Corona, J, Familial comedones. Evidence for autosomal dominant inheritance, Arch Dermatol 114 (1978) 1807–1809.
140. Rodin, HH; Blankenship, ML; Bernstein, G, Diffuse familial comedones, Arch Dermatol 95 (1967) 145–146.
Pseudoepitheliomatous hyperplasia






