Chapter 12 Treatment of infection in burns
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Infection control best practice
The microorganisms initially populating the burn wound represent a mixture of endogenous resident flora and airborne contaminants seeded by contact with the environment and attending personnel. Burn patients are immunosuppressed and should be protected from exposure to environmental contaminants. The most elaborate methods of isolation have failed to eliminate infection, although they have significantly reduced the incidence of cross contamination.1,2,3 The most effective means of reducing exposure of burned patients to exogenous bacteria is strict observation of appropriate hand washing by carers. Universal contact isolation is often routine in burn centers. Face masks, waterproof gowns and gloves should be worn whenever direct contact with body fluids and wound exudates is possible, thereby protecting both the patient and the carer from inadvertent contamination. All dressing materials should be maintained as patient specific. Intravenous pumps and poles, blood pressure devices, monitoring equipment, bedside tables and beds should be cleaned on at least a daily basis with antibacterial solutions. Terminal cleaning, following the discharge of the patient, should include the walls, ceiling, baseboards and floors. Mattresses should be covered with vinyl or other impermeable surface that allows culturing and cleaning without soiling, and be frequently inspected for cracks in their surfaces. At our institution we use Hepa air filters with 99.99% efficiency on 0.3 µm sized particles. They are changed regularly and cultured, if clinically indicated, by infection control monitoring. Most units now house major burn patients in individual, self-contained positive-pressure isolation rooms. However, common areas exist even within these units, mainly the bathing or showering facilities. These areas should be conscientiously cleaned between patients with an effective bactericidal agent specifically directed at the bacteria common to an individual unit. Disposable liners for cleaning surfaces are encouraged, and sterilizable instruments should be used for debridements.
Pathophysiology of the burn wound
Bacteria of normal endogenous skin flora are resistant to heat injury in practically the same degrees as are the skin cells. The bacteria on the surface are heat killed, as are the tissue cells of the surface, and initial cultures are usually sterile. The bacteria in the hair follicles and sebaceous glands may survive (depending on the extent of the burn injury), and the quantitative counts of biopsied specimens may show the same numbers of bacteria per gram (103) as found in the tissue prior to burning.4,5,6 The mean cell generation time in optimum conditions is approximately 20 minutes. Therefore, a single bacterial cell can increase in numbers to over 10 billion within a 24-hour period.7 As these bacteria increase in number following the thermal injury and reach levels of >105 bacteria per gram of tissue, they will erupt from the hair follicles and sebaceous glands and begin transmigrating over the injury, colonizing the dermal–subcutaneous boundary. If the burn is left untreated, perivascular growth is accompanied by thrombosis of vessels and necrosis of any remaining dermal elements, potentially transforming partial-thickness burns to full-thickness ones. As the levels of bacterial growth increase, so does the incidence of invasion of viable tissue and septicemia.8,9 Histologically, invasive infection is seen with bacteria in unburned tissue. Other signs of invasion can be the presence of hemorrhage in unburned tissue, small-vessel thrombosis with ischemic necrosis of unburned tissue, and dense bacterial growth in the subeschar space. The usual progression of bacterial colonization as the days pass is from Gram-positive to Gram-negative. By the 21st day post burn 57% of burn wounds still open will be colonized with extended-spectrum β-lactamase Pseudomonas. The natural progression with multiplication of bacteria and deepening of the burn wound is routinely avoided with modern burn care. With a combination of cleansing, antimicrobial topicals, antibiotic dressings, adherent dressings, and early surgery, burn wound progression and infection are avoided in most burn patients. However, even in the presence of modern care, early exposure to a virulent pathogen following injury can quickly progress to invasive burn wound infection and necrotizing soft tissue infection. Awareness of the circumstances of the burn and the natural evolution of a healing or surgically treated burn is critical to keeping patients safe.
Infection and the burn wound
Definitions for the burn patient
The Society of Critical Care Medicine (SCCM) and the American College of Chest Physicians (ACCP) have developed and reported on definitions for the systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock in the United States.10–12 Similar definitions were adopted at an international forum. This important effort has been made to help to standardize the diagnosis and management of patients, which is fundamental to clinical study and the advancement of care. Unfortunately, owing to the nature of burn injury, the established definitions have been found to be inadequate to describe the burn patient. Severe burn injury is associated with the most profound response to injury seen in any disease state. The hypermetabolism seen with burn injury can, in many ways, mimic the SIRS response. This hypermetabolism is a natural part of the body’s compensatory mechanism in response to burn injury and can last for up to a year following injury. Therefore, in 2007, a consensus conference was formed within the American Burn Association (ABA) tasked with authoring definitions of sepsis and infections in burn injury.13 The methodology of the conference was not unique to this committee, but rather has been broadly used in many disciplines in medicine, including the SCCM and ACCP in the development of the general definitions of SIRS and sepsis. In this section the discussion offered on SIRS, sepsis, burn wound infection, pneumonia, bloodstream infection, catheter-related bloodstream infection and urosepsis are in keeping with the consensus definitions put forth in that publication.
Burn wound erythema
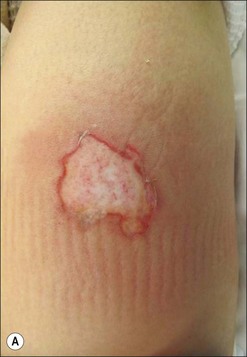
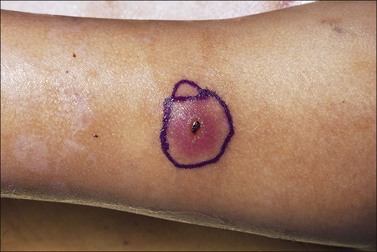
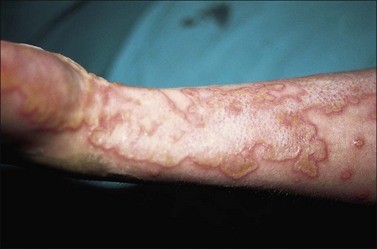
The most common burns are smaller than 10% of the total body surface area (TBSA). When presented early for treatment these smaller burns are typically clean. The presence of fever alone does not indicate infection, as burn is associated with an elevation in the body temperature set point. Similarly, fever frequency or severity is not proportional to burn size or depth. Fever can accompany burns of any size. To evaluate a burn for possible infection and determine a need for antibiotics, the examiner must be aware of the phenomenon of burn wound erythema. Burn wound erythema is defined as a redness surrounding the burn injury that is not a first-degree burn, and is not infectious. It normally appears 2–3 days following injury and dissipates by post burn day 5 or 6. It is likely related to the liberation of cytokines and other mediators of inflammation. In contrast to true infectious cellulitis, burn wound erythema is normally non-tender to palpation. Figure 12.1 demonstrates burn wound erythema.
Burn wound impetigo/graft ‘melting or ghosting’/ folliculitis / persistently open donor sites
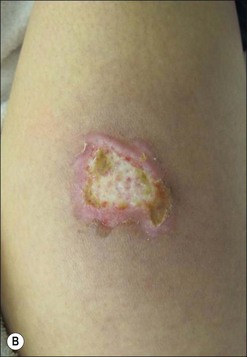
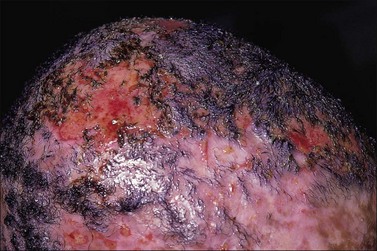
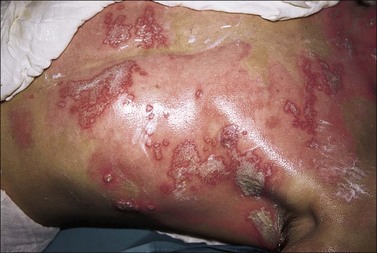
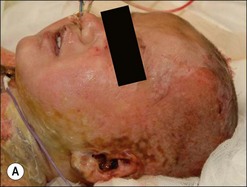
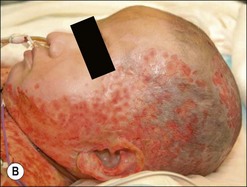
Burn wound impetigo, graft melting or ghosting, which is loss of a previously present skin graft (Fig. 12.2), and folliculitis (Fig. 12.3) are conditions that are well known to the burn care clinician. However, these clinical problems were not addressed specifically in the definitions put forth in the burn care consensus conference on infection and sepsis. These conditions are all most likely the result of bacterial colonization of the wound. Burn wound impetigo has been described as small multifocal superficial abscesses, which can cause extensive destruction of previously healed split-thickness skin grafts and donor sites (Fig. 12.4).6 This characteristic has led some to call the phenomenon graft melting or ghosting. On the scalp, a very similar problem occurs that is termed folliculitis. It is not certain whether these different clinical entities represent one or multiple disease processes pathophysiologically. They are grouped here for their similarities in clinical presentation and treatment. Cultures commonly reveal Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA). Treatment consists of twice daily cleansing with surgical detergent disinfectant, unroofing any abscesses, and twice daily application of a topical antimicrobial such as silver nitrate, Dakin’s solution, or most commonly mupirocin (N3). With folliculitis, trimming or shaving of the hair is also helpful.
Burn wound colonization
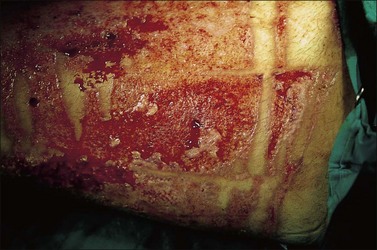
Burn wound colonization (Fig. 12.5) as recently defined, is ‘bacteria present on the wound surface at low concentrations. No invasive infection. Pathologic diagnosis: <105 bacteria per gram of tissue’.13 Clinically, the eschar or pseudoeschar may have a deteriorated or ‘infected’ appearance. No surrounding cellulitis is noted. Treatment consists of continued burn care with cleaning, topical antimicrobials, and surgery. If serial colony counts have been performed and an increase in colony counts has been noted, a change in topical agent may be advised.
Burn wound infection
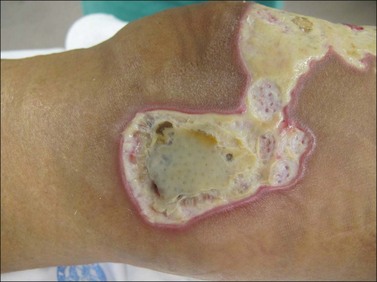
Burn wound infection is defined as ‘bacteria present in the wound and wound eschar at high concentrations. No invasive infection. Pathologic diagnosis: >105 bacteria per gram of tissue’. Clinically, the presence of cellulitis is the foundation of the diagnosis. Cellulitis involves advancing erythema, warmth, and tenderness (Fig. 12.6). The early burn with surrounding blanching erythema likely has an area of surrounding first-degree burn or burn wound erythema, and therefore no antibiotics are indicated. However, the burn wound with surrounding clinical cellulitis may have an atypical history or a late presentation. Often pathologic colors and odors alert the clinician to the strong likelihood of infection. Usual treatment of the infected burn wound consists of thorough cleansing, application of topical antimicrobials, and systemic antimicrobials. The offending organism is usually a drug-sensitive staphylococcus, and antimicrobials are started appropriately. Further choice of antibiotics and duration of treatment can be made by culture and sensitivity results, or merely by clinical progress of the wound. If the circumstances, appearance, or comorbidity history make consideration of Gram-negative pathogens reasonable, expansion of coverage is indicated. Occasionally, the severely neglected wound can progress to full thickness and even have a contained area of pus beneath a true eschar. Clinical suspicion for this situation arises when a desiccated eschar is encountered with the impression of fluctuance on examination. Such an area needs to be opened like any other abscess. This can be accomplished through a cruciate incision or partial sharp removal of the eschar. Special care should be taken with the evaluation of burn wounds in the elderly and diabetics, as the inflammatory response can be blunted and the wound severely underestimated.
The burn wound and toxic shock syndrome
One area of special consideration when caring for the small burn is the complication of toxic shock syndrome (TSS). TSS is a form of severe soft tissue infection (SSTI). In the burn wound this syndrome results from colonization with TSS toxin-1-producing Staphylococcus aureus. This disease is primarily of the young child, with a burn of <10% TBSA that would normally be thought to heal without problems. The incidence has been reported at approximately 2.6% with a mean age of 2 years. Clinically it is characterized by a prodromal period lasting 1–2 days with pyrexia, diarrhea, vomiting and malaise. A rash is often present, but at this stage the burn appears clean. Shock then develops in untreated cases. This is the time of maximal illness and is usually 2–4 days after the burn injury. Once shock has developed, mortality can be as high as 50%. The main defense against the development of this condition is knowledge and aggressive treatment. Since MRSA has emerged as the most common identifiable cause of severe SSTI, initiation of empiric anti-MRSA antimicrobials is warranted in all cases of suspected TSS.14,15
Invasive burn wound infection
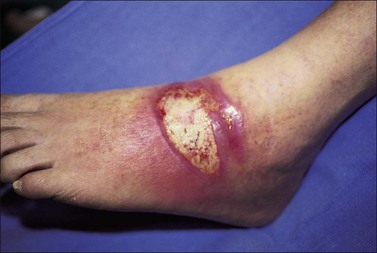
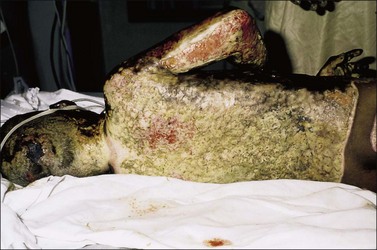
Invasive burn wound infection is ‘the presence of pathogens in a burn wound at concentrations sufficient in conjunction with depth, surface area involved and age of patient to cause suppurative separation of eschar or graft loss, invasion of adjacent unburned tissue or cause the systemic response of sepsis syndrome’.13 Invasive burn wound infection can occur without sepsis. However, many invasive burn wound infections are rapidly progressive, life-threatening, and require urgent surgical treatment as part of a spectrum of care to gain control of the wound (Fig. 12.7). The clinical diagnosis can be manifested by a change in appearance of the wound from pink and white to yellow, brown, black and green, with distinct odors. Time and some topical agents also can produce color changes that can be misleading. Therefore, it must be recognized that the most reliable sign of invasive burn wound infection is conversion of an area of partial-thickness burn to full-thickness necrosis, or the necrosis of previously viable tissue in an excised wound bed (Fig. 12.8). There are other reasons for burn wound deepening and conversion, and these must be considered. Once an invasive infection is established, progression can be very rapid. Hemorrhagic bullae, satellite lesions and previously normal skin go from erythematous to necrotic and are characteristic of an aggressive Gram-negative pathogen, usually Pseudomonas species (spp). Such lesions are termed ecthyma gangrenosa (Fig. 12.9).
Treatment of invasive burn wound infection is the elimination of all dead tissue, including aggressive removal of dead muscle if present. Here the surgeon must be as aggressive as in necrotizing fasciitis, if the wound is to come under control and eventually support grafting. Surgical control can mean conversion of a tangential excision to fascial level, or amputation where previous attempts were being made to spare a limb. Dead muscle in particular can be problematic as compromised areas can be hidden from view. In the larger burn, any burden of dead tissue with loss of the skin barrier, combined with immunosuppression, makes a very aggressive surgical approach mandatory. Often the initial operation to gain control of an invasive burn wound infection may conclude with no wound coverage other than soaking dressings, with a plan to return to the operating room in the near future. The empiric antimicrobial choice should be broad coverage for fungi, drug-resistant Gram-positive and -negative organisms. Once pathogens are identified and sensitivities reported, antimicrobial management follows this information with persistent efforts to cover the offending organisms with adequate well-timed doses of effective antibiotics chosen for their specific activity against the pathogen. This requires a coordinated effort from clinician, microbiologist and pharmacist. The treatment of the surface is aggressive also. Following the complete removal of dead tissue, topical antimicrobial soaks are commonly used, including silver nitrate, sulfamylon, and Dakin’s solution.16 Each can be used with topical nystatin to improve kill of fungus if this is a concern. In association with this, increasing the change frequency of the soaking dressing to four times a day may be warranted.
Extremely virulent pathogens
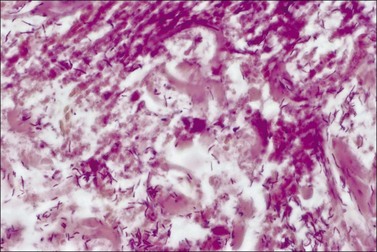
Amphotericin-resistant Fusarium has the ability to invade living tissue and create a wavefront of necrosis. This pattern is histologically recognizable by the presence of fungal hyphae extending like fingers to the border between necrotic and intact tissue, and represents dangerous invasion of viable tissue. Very aggressive removal of all dead tissue, and voriconazole administered systemically along with the use of topical antifungals, can be successful against this virulent organism. A similar virulent profile is possessed by Aspergillus and Mucor spp. Candida colonization will be present in 30% of burn patients with burns >40% during their hospital stay. It is for this reason that nystatin was added to topical silver sulfadiazine. This change has resulted in a marked decrease in three-organ involvement with Candida, which is usually required for the diagnosis of candidal sepsis (Fig. 12.10).17 Serum antibody titers to Candida have recently been advocated to assist in the early diagnosis.18 Pre-mortem diagnosis, confirmed with sufficient time to implement appropriate treatment, occurs in <40% of infected patients.19 Because of their relatively large size, Candida are usually filtered out of blood at the capillary level; therefore, arterial blood cultures are recommended. Organisms are usually seen in the urine when sepsis from a distant site occurs. Candidal sepsis is considered when C. albicans can be isolated from any three of the following tissue sites: blood, wound, urine, bronchial washings, or by a positive retinal examination. Candidal spores are omnipresent in the burned patient, appearing in stools, nasopharynx swabs, urine samples and cultures of intact integument. However, fewer than 20% of patients with candidal wound colonization develop widespread candidiasis.20 Only when the controlling bacterial populations are eradicated by systemic antibiotics or the host becomes immunosuppressed does candidal infection occur, frequently following a bacterial sepsis. There is a 3–5% incidence of candidemia in the burned population17,20–22 and a comparable rate of burn wound invasion.21 Candidal infections occur most commonly in patients with large burn injuries who are hospitalized for long periods and have received multiple courses of antibiotics. Prophylactic treatment with oropharyngeal and topical nystatin has therefore become recommended,17 although fluconazole may be more effective in some cases. Mortality associated with Candida is usually from amphotericin-associated renal failure.
Culturing of the burn wound
Daily inspection of the burn wound by a burn surgeon is mandatory for decision making. Major burn wounds usually become colonized or infected within 3–5 days after admission. Often, infection arises from the patient’s own bacterial flora and not from an exogenous source. Burn wound biopsies should be taken from any area of the wound that has changed in appearance. If a simultaneous sample is examined by a pathologist, a diagnosis of burn wound invasion in need of emergency surgical treatment can be made. Quantitative cultures showing high bacterial counts correlate with histologic evidence of burn wound infection in approximately 80% of cases.6,23,24 If quantitative biopsies reveal >103 organisms/g of tissue, a change in topical therapy is indicated. If bacterial counts exceed 105/g of tissue, localized burn wound infection should be considered and a histological examination performed. If histological evidence of invasion is present, systemic antibiotics should be given and the wound should be excised.6,15,25 Bacterial sepsis is often heralded by changes in the color, odor or amount of exudate from the wound, whereas fungal invasion is clinically suggested by a rapidly emerging and spreading dark discoloration. Consequently, the burn wound must be closely assessed for these manifestations.
There is considerable practice variation in the more routine use of wound cultures. The literature from Robson et al. in 1973 demonstrated that burn wound colony counts >105/g tissue led to only a 19% graft survival rate, whereas colony counts <105/g tissue had a 94% chance of graft survival.26 This led to widespread use of burn wound biopsies and cultures. In 1996 Steer et al. demonstrated a wide variation in bacterial densities within a wound at the same moment.27 This information brought into question the value of clinical management change based on routine burn wound culturing. In 2003 Barret and Herndon looked at the effect of burn wound excision on bacterial colonization and invasion.28 They noted that even preoperative colony counts of >105 were reduced to <104 when wounds were treated aggressively through surgery. As a result of this type of treatment, skin graft take was excellent. This is in contrast to Robson’s findings of 40 years earlier. The change is likely related to the change in surgical paradigm and technique. Interestingly, in the Herndon and Barret study, a subgroup was identified with preoperative colony counts >106/g tissue. This group had post-excision pregrafting cultures that averaged 104/g tissue, but they suffered a 75% infection rate and graft loss. It is clear that burn wound colony counts have significant value in guiding the care of the patient. However, these counts may also possibly be unreliable and inaccurate because of sample site variation. Box 12.1 gives a list of reasons and uses for burn wound cultures.
Box 12.1
Burn wound cultures in burn care
1 Early cultures should be negative or have low counts, with sensitive gram positive organisms. Therefore, positive cultures or high counts suggests early contamination of the burn wound.
2 If an invasive burn wound infection is suspected, wound culture and histologic analysis can aid in confirmation of the clinical diagnosis.
3 Routine culturing and identification of colonization may aid in empiric antimicrobial coverage if the patient subsequently becomes ill.
4 Increasing colony counts may indicate a need to change topical antimicrobial agents.
5 Not all organisms are created equal. Wound colonization with particularly virulent or resistant organisms may be a predictor of impending invasive burn wound infection.
6 Operative wound colony counts of >106 suggest a high risk of infectious complication and graft failure.
7 Burn wound culture results may aid in the evaluation of nosocomial spread of organisms and guide infection control practice.
Viral infection of the burn wound
In recent years more attention has been turned to the diagnosis and treatment of viral infections in burned patients. Prospective and retrospective assays of sera have documented a large incidence of subclinical viral infection. In one of the first large retrospective studies from the 1980s, Linnemann et al. assayed stored sera of burned children.29 A fourfold increase in antibodies to cytomegalovirus (CMV) was found in 22% of patients, 8% had increased herpes simplex titers, and 5% demonstrated a rise in varicella zoster titers. The study continued in a prospective manner, with 33% of the children developing CMV infection, 25% developing herpetic infection, and 17% developing adenovirus infection.29
Cytomegalovirus infection frequently occurs concurrently with bacterial and fungal infections, but rarely alters the patient’s clinical course. Primary CMV infection or reactivation of CMV was reported with an overall frequency of 33%.29 Importantly, in that study, prospective analyses ‘directly correlated CMV infection with more severe burns, more skin grafts, and subsequent higher numbers of blood transfusions.’ The clinical manifestation rarely occurs in patients with <50% TBSA burn infected, and typically presents approximately 1 month post burn as a fever of unknown origin and lymphocytosis.29 Rennekapff et al., in a 2006 review, identified a seroconversion rate of between 18 and 22% for burn patients who were seronegative for HCMV prior to their burn injury.30
Pathophysiologically, CMV inclusions may be identified in the cells of multiple organs but have not been reported in the burn wound.31 Immunocompromised patients have a higher frequency of CMV infection, which results in a broad range of adverse conditions ranging from febrile illness to systemic infections with organ (e.g., lungs, brain, liver, colon, pancreas) involvement.32 CMV infection has also been associated with unexplained fever and lymphocytosis based on a concomitant rise in specific antibodies.29,31 More recent studies have suggested that up to 23% of seronegative patients with severe burns seroconvert, while more than 50% of seropositive patients reactivate CMV based on a fourfold or greater rise in antibody titer.33,34 The fact that only two reports of systemic CMV infection in severely burned patients were published suggests33,35 that systemic disease is an unusual occurrence and that the majority of patients who demonstrate increased CMV-specific antibody sustain more limited CMV infections. The absence of reports that account for the frequency of severely burned patients with increased CMV antibodies suggests that most of these infections are subtle and have been overlooked by past studies. Generalized, non-healing burn wounds have been associated with CMV pathology in endothelial and periendothelial cells.33 Inclusion bodies consistent with CMV infection, as well as CMV antigens, were detected by immunohistochemical staining of a skin biopsy from a transplanted cadaver allograft (from a CMV-positive donor) on a severely burned adult male, but the relationship of the CMV infection to necrosis, inflammation and increased vascularity evident in the infected skin is not known.36 Results from animal experiments demonstrated that severe burn injury predisposes to murine CMV infection, and murine CMV infection was associated with increased susceptibility to sepsis.37,38
The burned patients who most commonly contract the infection are known to have received multiple blood transfusions, which represent a major source of contamination. In addition, it has been shown in an animal model that cadaver skin has the ability to transmit CMV infection. In a recent study by Tennenhaus et al.39 US and German burn centers were surveyed and evaluated for awareness, perceptions, diagnosis, and treatment of CMV in patients with burn injury. CMV infection incidence was reported at 1 : 280 in German and 1 : 870 in US burn centers. When testing, 70% of German and 19% of US burn centers used serology; 52% German and 25% US centers used body fluid viral isolation; and 43% German and 6% US centers used leukocyte CMV-DNA analysis. Two-thirds of the German and half of the US centers distinguished infection from disease. A total of 43% German and 19% of the US centers would treat the established disease.
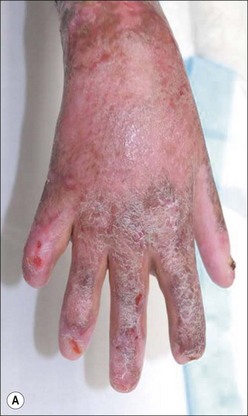
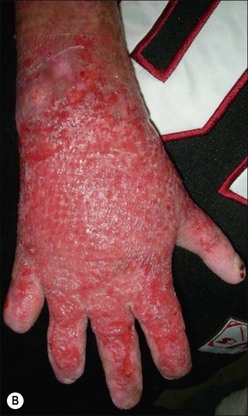
Herpes simplex infections most commonly manifest themselves as vesicles in healing partial-thickness burns or split-thickness donor sites (Fig. 12.11). In the immunocompromised burn patient, however, the infection usually starts with barely noticeable red macules, which then progress rapidly and spread over entire donor sites and previously healed areas. A near-total loss of epidermal coverage can occur (Fig. 12.12a,b and Fig. 12.13a,b). Other epithelial surfaces such as the oral or intestinal mucosa can also be involved, potentially causing erosion and perforation. The clinical manifestations of lesions may be preceded by unexplained fever unresponsive to routine antibiotic coverage.40 In recent years, an increased mortality, extensive visceral involvement, and necrotizing tracheobronchitis have been associated with herpetic infections after burns. A retrospective study characterizing the incidence, presentation, and outcome of 14 patients with facial herpes rashes out of 95 severely burned intubated adults was performed by Fidler et al., showing that rashes attributed to herpetic infections were found in at least 15% of patients, but no difference in mortality or length of stay could be observed between patients with or without the infection.41 The authors also noted that the course of this infection was relatively benign in this group of acyclovir-treated patients. Partial-thickness burns and donor sites infected with herpes may convert to full-thickness injuries, requiring skin grafting for ultimate closure. Necrotizing hepatic and adrenal lesions may lead to multisystem organ failure. Mortality in patients with disseminated infection is about twice that expected for patients of similar age and burn size. Split-thickness grafts provide adequate coverage of previously infected herpetic wounds,42 but the coverage is frequently associated with secondary graft loss and the need for reoperation and patch grafting.
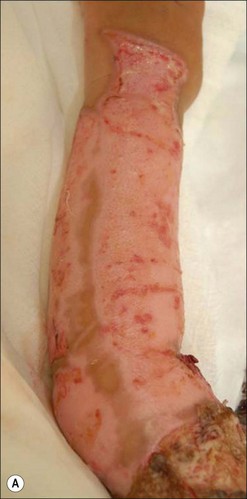
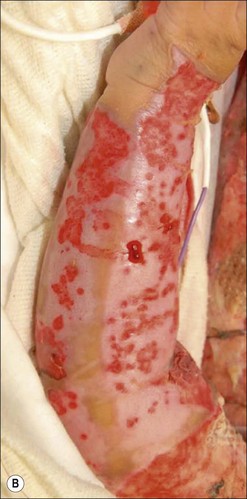
Figure 12.13 Same patient as in Figure 12.12. (a) The left arm, which was used twice as a donor site for split-thickness skin graft (days 4 and 11). The image is from day 17, with initial manifestation of herpetic infection with red macules. (b) Day 18 post burn; 30 hours later, conversion to confluent defects with near total loss of epidermis.
Chickenpox (varicella zoster) infection is a frequent occurrence in school-aged children and is rapidly spread through inhalation of the virus. Varicella infections can be life-threatening in an immunocompromised host and small epidemics have occurred within pediatric burn units.42 The characteristic fluid-filled lesions appear in healed or healing partial-thickness burns, as well as uninjured epithelium and mucous membranes. Owing to the fragility of the newly healed or healing skin, the vesicles are much more destructive in the injured than in the uninjured skin, and may present as hemorrhagic, oozing pockmarks, which are prone to secondary infection and subsequent scarring. Neovascularized skin grafts may be lost, and further grafting procedures should be delayed until the lesions are quiescent.
Burn-associated infections
Sepsis
Rapid and complete closure of deep burns is the best defense against the development of sepsis in the burn patient. If a comparison is made between burn patients of equal size, those who have an occurrence of sepsis during their hospitalization will have decreased lean body mass and increased mortality. A major focus of rounds in the burn intensive care unit is on the identification of sepsis. Sepsis was addressed at the American Burn Association Consensus Conference to Define Sepsis and Infection in Burns.13 Below is the definition as outlined in the publication:
The trigger includes at least three of the following: