Transplantation Biology and Applications to Plastic Surgery
Damon S. Cooney
Justin M. Sacks
Gerald Brandacher
W. P. Andrew Lee
The transplantation of tissue from one location to another is a fundamental concept in plastic surgery. It is not surprising that the first successful transplantation of tissue from one person to another in the form of a kidney transplant was performed by a plastic surgeon, Dr. Joseph E. Murray. Other pioneering plastic surgeons helped spawn the new field of allogeneic organ transplantation, and with the development of improved surgical techniques and modern immunosuppression, transplantation has become the treatment of choice for end-stage organ failure of the liver, heart, lung, pancreas, and kidney. It is fitting that transplantation has returned to the field of plastic surgery more than 50 years later with the development of reconstructive transplantation, the new era in transplant medicine. It is only within the last decade that transplantation of vascularized composite allografts (VCAs), such as hand and face transplants, has become a clinical reality. VCAs involve transplantation of composite structures for reconstructive surgery and thereby fulfill a prime mandate of plastic surgery: to replace and restore devastating tissue defects using “like-with-like.” The ability to transfer vascularized allografts through microvascular surgical techniques, restoring form and function for complex cutaneous and musculoskeletal defects, is revolutionizing the field of reconstructive surgery and has added another rung to the “reconstructive ladder.” Long-term allograft survival, however, can only be achieved, as for any solid organ transplant, through the use of systemic immunosuppression with its associated sequela of organ toxicity, opportunistic infections, and potential for malignancy. Current research on immunomodulation and induction of tolerance holds promise for reducing the need for long-term high-dose immunosuppression. Although reconstructive allotransplantation in humans is a relatively new area with small numbers of patients, there are reasons to think that new innovations in immunomodulation and tolerance may come from the field of VCAs. For example, reconstructive transplant patients usually do not suffer from life-threatening illness or comorbidities and therefore the impetus to minimize side effects from immunosuppressive medications is stronger. Also, the ability to directly and continuously observe transplanted tissue that includes a skin component allows for the use of novel experimental protocols as rejection is seen earlier and can potentially be reversed by topical agents. Finally, the presence of vascularized bone marrow in many VCA grafts raises the possibility of unique modulatory strategies, as will be discussed.
INTRODUCTION
Human tissue transplantation is an ancient concept. According to legend from the fourth century AD, Saints Cosmos and Damian—twin brothers—replaced the gangrenous leg of a parishioner with the leg of a deceased Ethiopian Moor. The earliest reliable, documented outcomes of allogeneic and xenogeneic skin grafts in human recipients were published by Schone in 1912 and Lexer in 1914. Schone and Lexer demonstrated that these grafts did not survive more than 3 weeks after transplantation. Padgett provided further evidence in 1932, when he reported rejection of all skin allografts within 35 days in 40 patients. However, Padgett also demonstrated that skin grafts exchanged between identical twins survived indefinitely. World War II accelerated progress in allotransplantation. Gibson, a plastic surgeon at the Glasgow Royal Infirmary, described the accelerated rejection of skin grafts in pilots due to the presence of humoral antibodies after repeat exposure to the same donor, also known as the “second-set rejection.” Medawar joined Gibson to investigate this phenomenon and, in combination with Billingham and Brent, laid the foundation for modern immunology. In 1954, Dr. Joseph E. Murray and colleagues performed the first successful kidney transplant between identical twins. Furthermore, the introduction of a novel immunosuppressive drug, 6-mercaptopurine and its precursor azathioprine (AZT) by Charles Zukosi and Roy Calne in 1960, was responsible for improvements in the field of organ transplantation.
With the discovery of the first human leukocyte antigen (HLA) in 1958, the matching of tissue beyond simply matching blood types became possible. Knowledge of these antigens allowed the avoidance of graft-versus-host disease. The first successful human bone marrow transplant was performed in 1968. A 4-month-old boy who had Wiskott-Aldrich syndrome received a bone marrow transplant from his sibling that effectively restored his immune system, duplicating Medawar’s animal findings that had previously resulted in immune tolerance in chimeric mice. Medawar’s chimeric mice contained genetically distinct cells originating from separate and unique zygotic cells.
Coinciding with these first successful human bone marrow transplantations, all major components of human clinical allotransplantation including immunosuppression, tissue preservation and matching, and complex microvascular techniques were elucidated. Following the first kidney transplantation, other solid organs such as the heart, liver, lung, and pancreas were transplanted and nonspecific immunosuppressive agents such as cyclosporine A (CsA) and FK506 were developed.
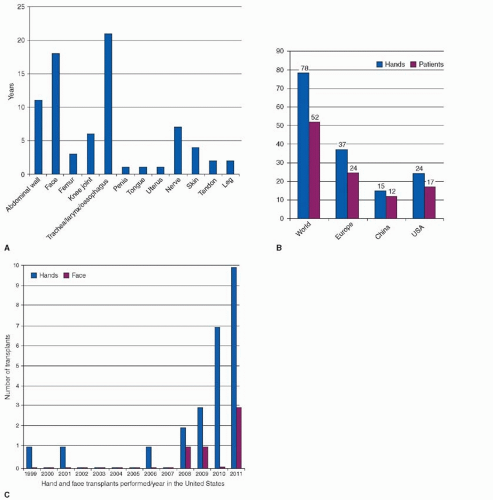
In the last two decades, over 150 different VCAs including more than 80 upper extremities and 24 partial faces, as well as abdominal walls, larynx, tongue, uterus, penis, vascularized bone and joint, and individual tissue components like peripheral nerve, flexor tendon, and skin have been successfully transplanted using conventional immunosuppressive protocols. Of the upper extremity transplants performed to date, only one graft was lost while patients were on high-dose immunosuppression. In the combined American and European experience, the early to intermediate allograft survival is greater than 95% (Figure 6.1).
Current immunosuppression protocols developed within the last century have allowed these transplantation ideas to become a surgical reality. The risk/benefit ratio that must be optimized when transplanting a piece of tissue that optimizes form and function but does not preserve or prolong life poses an ethical dilemma. Exposure to life-long immunosuppression comes with risk that must be articulated to the patient. Current immunologic research focuses on ways to obviate the use of systemic immunosuppression for both solid organ and reconstructive transplantation procedures. The field of reconstructive transplantation will become ubiquitous once tolerance can be achieved. Currently, great strides are being made in large animal studies and in the first clinical trials, moving ever closer to elucidating the immunologic processes that will unlock these barriers and allow the next revolution in plastic surgery to begin.
NOMENCLATURE
Proper nomenclature will help clarify subsequent discussions. Certain terms such as transplant, flap, and graft are often used to refer to a VCA. However, these terms should be used carefully with their true meaning in mind to ensure clear communication.
Transplantation can be defined as the transfer of tissue or an organ to another person or to a different location in the same person. According to this definition, much of what reconstructive surgeons do can be classified as a type of transplant. However, in its usual medical usage, the term transplant is used to describe an allotransplant or tissue transferred from a living or deceased human donor to another genetically not identical human patient. Within this chapter, our discussion of transplantation will be focused mostly on the topic of allotransplantation, as the transfer of autologous tissue locally or distantly is covered elsewhere in the text.
A graft is tissue completely separated from its donor bed and moved to a separate recipient bed, its survival relying on ingrowth of new vessels from the surrounding recipient tissue (Chapter 1). A vascularized graft or flap either remains attached to its native blood supply or becomes revascularized via microvascular anastomoses to recipient vessels (aka free flap). An autograft refers to tissue transplanted from one location to another within the same individual. An isograft is tissue transplanted between genetically identical individuals, such as transplants between syngeneic mice or human monozygotic twins. An allograft or homograft is tissue transplanted between unrelated individuals of the same species. A xenograft or heterograft is tissue transplanted between different species.
Transplantation can also be described according to the site into which the tissue is transferred. An orthotopic transplant is transferred into an anatomically similar site, whereas a heterotopic transplant is transferred into a different site from its donor
origin. The term reconstructive transplantation is used to differentiate the transfer of composite tissues such as the hand or face from more traditional solid organ transplants. During the development of reconstructive transplantation, this novel field was commonly referred to as composite tissue allotransplantation (CTA). Unfortunately, the use of this term has caused some confusion and has largely fallen out of favor. In particular, the use of the word tissue raised the concern that reconstructive transplantation could be confused by regulatory bodies (such as the FDA) with non-vascular tissue transplantation, with potentially negative regulatory consequences. Therefore, vascularized composite allograft (VCA) transplantation has supplanted the term CTA to avoid this confusion.
origin. The term reconstructive transplantation is used to differentiate the transfer of composite tissues such as the hand or face from more traditional solid organ transplants. During the development of reconstructive transplantation, this novel field was commonly referred to as composite tissue allotransplantation (CTA). Unfortunately, the use of this term has caused some confusion and has largely fallen out of favor. In particular, the use of the word tissue raised the concern that reconstructive transplantation could be confused by regulatory bodies (such as the FDA) with non-vascular tissue transplantation, with potentially negative regulatory consequences. Therefore, vascularized composite allograft (VCA) transplantation has supplanted the term CTA to avoid this confusion.
TRANSPLANT IMMUNOLOGY
Transplantation Antigens
Transplantation of organs or tissues between genetically disparate individuals of the same species (allogeneic individuals) leads to recognition and rejection of the allogeneic tissue by the recipient’s immune system. This “alloimmune response” that discriminates between self- and non-self tissues remains the main barrier to successful transplantation. These immunologic responses are initiated by graft antigens that are genetically encoded polymorphic proteins. The result of this interaction determines the acceptance or rejection of allograft tissue. For this reason, learning how to suppress these responses is a major goal of transplant immunologists. Transplant tolerance, as discussed, can be mediated by central or peripheral mechanisms and can be acquired with the assistance of either immunosuppression or immunomodulation.
Antigens are cell surface glycoproteins that are encoded in the major histocompatibility complex (MHC), a multigene cluster located on chromosome 6 in humans. There are two classes of MHC molecules, class I and II, that differ in their structure, function, and tissue distribution. MHC class I antigens are expressed on all nucleated cells, whereas class II expression is restricted to antigen presenting cells (APCs), such as B lymphocytes, monocytes, macrophages, dendritic cells (DCs), endothelial cells, and activated human and rat T cells. In humans, the MHC antigens are known as HLA. Each individual has two MHC regions, one of paternal and one of maternal origin. Each MHC contains an inherited group of HLA genes or haplotypes: HLA class I genes known as HLA-A, -B, -C and HLA class II genes known as HLA-DR, -DP, and -DQ. The HLA antigens determine the compatibility of all organ and tissue transplants.
Allogeneic Transplantation
Billingham, Brent, and Medawar demonstrated in their seminal 1953 Nature article that neonatal mice and irradiated adult mice developed donor-specific tolerance to skin grafts subsequent to successful engraftment of splenic and bone marrow cells into the recipient. These animals were considered chimeras consisting of both donor and recipient T cells. This built the foundation for the concept that cell-based therapies in clinical transplantation could potentially modify the host immune system to allow minimization or even avoidance of pharmacological immunosuppression. Since then, immune tolerance has been the “Holy Grail” of transplantation research.
Rejection of transplanted tissue occurs through both cellular and humoral immune responses. These responses are generated when host APCs and T lymphocytes respond to the genetic differences in the MHC molecules expressed by the donor cells. T cells have fundamental roles in graft rejection, and their responses are rapid and vigorous and ultimately will lead to inflammation and tissue destruction. There are two main pathways by which host T cells recognize donor alloantigens. Following transplantation, donor APCs migrate toward host lymphoid tissues and can directly activate recipient T cells. Once T cells are activated, they become effector T cells and migrate to the graft and mediate rejection. This is aptly named the direct pathway of allorecognition. In contrast to the direct pathway, host APCs play a significant role in the indirect pathway of allorecognition where they present processed donor antigens to host T cells. Both pathways of allorecognition are important in mediating graft rejection; however, the indirect pathway is thought to be of greater significance in the physiology of chronic graft rejection.
DCs are the most efficient APCs and have the capacity to take up, process, and present antigens to T cells in vivo. DCs rapidly respond to inflammatory stimuli, microbial products, or alloantigens following transplantation and express high levels of MHC class II and costimulatory molecules essential for T-cell activation.
Immunosuppression
All allotransplant recipients require some form of immunosuppression. Without these immunosuppressive modalities, rejection would inevitably occur in individuals unless they were genetically identical (identical twins). The immunosuppression used for transplantation of VCAs has for the most part mirrored the regimens for solid organ transplantation.
Several pharmacological drugs are used to prevent and control graft rejection (Table 6.1). It is important to note that these drugs lack selectivity and cause generalized immunosuppression rendering transplant patients highly susceptible to opportunistic infections and certain types of malignancies. Based on their mode of action, there are four main groups of immunosuppressive drugs: (1) steroids, (2) cytotoxic/antiproliferative drugs, (3) anti-T-cell agents (calcineurin inhibitors), and (4) induction agents (polyclonal and monoclonal antibodies). One of the first immunosuppressant used was steroids with broad anti-inflammatory actions (i.e., prednisone and prednisolone). These medications inhibit activation of several transcription factors, thus modifying gene transcription and inhibiting cellular activation and cytokine production. Prednisolone was one of the first pharmacological agents used in allogeneic organ transplantation. Despite the well-known side effects of long-term use, steroids are still widely used today in combination with other immunosuppressive agents in most solid organ and VCA protocols. Short courses of high-dose steroids continue to be the frontline treatment for acute rejection episodes in all types of transplantation. Cytotoxic/anti-proliferative drugs include cyclophosphamide, methotrexate, AZT, and mycophenolate mofetil (MMF). These medications interfere with DNA replication and kill/arrest proliferating lymphocytes that are activated by alloantigens. Earlier, nonspecific anti-proliferative agents such as AZT had many side effects and increased the risk of transplant-associated malignancy. MMF has replaced the other agents in many protocols due to its ability to block purine synthesis selectively in T and B cells, which dramatically decrease side effects. Agents that selectively inhibit the activation pathways of T cells are typically fungal or bacterial products (i.e., CsA, tacrolimus [FK506], and rapamycin [RAPA; sirolimus]). CsA and tacrolimus inhibit the signaling pathways of T-cell activation by interfering with calcineurin activation and interleukin (IL)-2 gene transcription (Figure 6.2). CsA is a metabolic extract from the fungus Tolypocladium inflatum gamus described in 1976. Its discovery revolutionized the field of solid organ transplantation by significantly increasing the survival of kidney, heart, and liver allografts. CsA was shown to prolong limb allograft survival in experimental animal models and thereby encouraged clinicians to pursue VCA in the 1980s and 1990s. Tacrolimus is a macrolide lactone antibiotic isolated from soil fungus and also inhibits the calcineurin/IL-2 pathway of T-cell activation, although at a different point in the pathway from cyclosporine. It has a favorable
side-effect profile as compared with CsA, with less transplant-associated malignancy, although it has significant nephrotoxicity when used for long periods of time. Tacrolimus has replaced cyclosporine in many protocols for solid organ transplantation and has been a mainstay in all the clinical reconstructive transplantation treatment regimen. RAPA is an inhibitor of the mammalian target of rapamycin (mTOR), which in turn inhibits multiple biochemical pathways critical for cellular proliferation with the main target being T cells. RAPA is an attractive alternative to CsA and FK506, having a significantly different side-effect profile in particular with regard to its nephrotoxicity, promoting tolerance in some circumstances, and having anti-proliferative and anti-neoplastic properties. It does, however, suffer from the draw-back of having profound negative effects on wound healing that may preclude its use in the early postoperative period. These immunosuppressive agents have been reported to allow successful allogeneic transplantation in clinical solid organ transplantation and VCA such as extremity transplantation and face transplants with both high graft and patient survival rates.
side-effect profile as compared with CsA, with less transplant-associated malignancy, although it has significant nephrotoxicity when used for long periods of time. Tacrolimus has replaced cyclosporine in many protocols for solid organ transplantation and has been a mainstay in all the clinical reconstructive transplantation treatment regimen. RAPA is an inhibitor of the mammalian target of rapamycin (mTOR), which in turn inhibits multiple biochemical pathways critical for cellular proliferation with the main target being T cells. RAPA is an attractive alternative to CsA and FK506, having a significantly different side-effect profile in particular with regard to its nephrotoxicity, promoting tolerance in some circumstances, and having anti-proliferative and anti-neoplastic properties. It does, however, suffer from the draw-back of having profound negative effects on wound healing that may preclude its use in the early postoperative period. These immunosuppressive agents have been reported to allow successful allogeneic transplantation in clinical solid organ transplantation and VCA such as extremity transplantation and face transplants with both high graft and patient survival rates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree