Topical Retinoids in Ethnic Skin
Stefani Takahashi
Julie Iwasaki
Vitamin A (retinol) and its derivatives are known as retinoids.1 Their topical forms have been used for acne, photoaging, and hyperpigmentation for decades. Throughout the years, many new formulations and vehicles have become available, and continued research can help improve their use. This chapter will discuss all-trans retinoic acid, all-trans retinol, adapalene, and tazarotene, along with their pharmacology, indications, and side effects. Additional focus will be given to the use of such topical retinoids in ethnic skin.
Topical Retinoids All-trans Retinoic Acid
All-trans retinoic acid (tretinoin), a metabolite of retinol, was developed more than 30 years ago and initially was found to loosen comedones. Thus, it was the first retinoid used for the topical treatment of acne. Ortho Pharmaceuticals introduced this drug as Retin-A in the 1970s. Women using Retin-A for acne coincidentally discovered that it also improved their skin texture, even after their acne was under control. Retin-A was then found to have a new application: to improve photoaging by improving fine lines and enhancing general skin appearance.
As research continued, additional formulations of tretinoin became available. One formulation was Renova, a tretinoin in a new emollient vehicle, which was approved by the FDA to help improve photodamaged skin. Because of the well-known drying properties of Retin-A, Renova was developed to counteract this side effect. Its moisturizing quality was better tolerated in postmenopausal women. Avita is a tretinoin formulation that complexes with polyoprepolymer-2 to slow its absorption into the skin, which decreases irritation. Retin-A Micro uses a microsponge technology to deliver tretinoin in a more controlled manner to similarly reduce irritation.
All-trans retinol
All-trans retinol is the parent form of vitamin A so it is not considered a drug when it is added to other compounds. In vitro studies have shown that it is oxidized in the skin to tretinoin; thus, it has similar side effects to this drug. Because it shares tretinoin’s irritant qualities, newer formulations were developed to allow for a more controlled delivery. all-trans retinol was introduced into cosmetic products by Avon in 1984 to improve photoaging and hyperpigmentation.2 It is available in formulations of numerous over-the-counter products.
Adapalene
Adapalene, a derivative of naphthoic acid, is commonly available as a 0.1% gel, cream, or lotion to be applied once daily. Adapalene microcrystals penetrate open follicles to the depth of the sebaceous glands within 5 minutes of treatment. Adapalene was found to have a more selective interaction with retinoid receptors than tretinoin; thus, it was developed to improve acne with less skin irritation. In the 1990s, Galderma released this drug as Differin.2,3
Tazarotene
Tazarotene, released by Allergan as Tazorac, is a topical retinoid developed to help both acne and psoriasis. The 0.1% tazarotene gel is FDA approved for use in both acne and mild-to-moderate psoriasis; the 0.05% tazarotene gel is only FDA approved for psoriasis. Its side effects include skin irritation and koebnerization. Tazarotene cream 0.1% has also been released by Allergan as Avage to help improve photoaging (facial fine lines, wrinkling, hypopigmentation, hyperpigmentation, and solar lentigines).
Pharmacology of Topical Retinoids
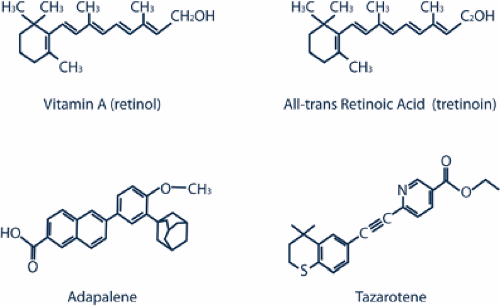
The first-generation topical retinoids, such as tretinoin, are made by modifying the polar end group and the polyene side chain of vitamin A. Adapalene and tazarotene are both third-generation polyaromatic retinoids that are made by cyclization of the polyene side chain1 (Fig. 10-1).
All-trans retinoic acid and all-trans retinol
Topical all-trans retinol is taken up by epidermal keratinocytes as a fat-soluble drug and binds to cellular or cytosolic retinol-binding protein (CRBP). Inside the keratinocytes, excess all-trans retinol is stored as retinyl esters in the form of lipid droplets by acyl CoA:retinol acyl transferase (ARAT). Because topical all-trans retinoic acid cannot be reduced to retinol, it increases the amount of intracellular retinoic acid to cause potential side effects. If retinoic acid levels become low in the epidermis, retinol is mobilized from retinyl esters and is oxidized to all-trans retinoic acid and its isomers (9- cis).
All-trans retinoic acid, bound to cellular all-trans retinoic acid-binding protein (CRABP), then binds to nuclear retinoic acid receptors (RAR), while the isomers bind to both retinoid X receptors (RXR) and RAR. RAR and RXR belong to the steroid-thyroid hormone family, and each family contains an alpha, beta, and gamma isotype. They form homodimers and heterodimers that bind to retinoic acid response elements (RAREs) in DNA to directly influence cell differentiation, proliferation, and immune responses (Fig. 10-2).
C-Jun and C-Fos are genes that are involved in photoaging. Levels of C-Jun rise when exposed to ultraviolet (UV) radiation, whereas C-Fos remains the same. C-Jun and C-Fos can then combine to form a heterodimer, activator protein-1 (AP1), which induces collagenase, gelatinase, and stromelysin. Retinoids inhibit the overexpression of C-Jun, which causes an indirect effect from the down-regulation of AP1 by competing for required coactivator proteins. AP1 is normally responsible for proliferative and inflammatory responses; thus retinoids have antiproliferative and anti-inflammatory actions.1,2,4
Adapalene
Adapalene is a lipophilic synthetic retinoid with a selective affinity for RAR-beta and RAR-gamma. RAR-gamma is the primary receptor for topically applied adapalene because RAR-beta is not present in epidermal keratinocytes. Only trace amounts of adapalene are absorbed systemically because adapalene’s lipophilicity causes selective uptake into the pilosebaceous unit and dissolution within the sebum. The hepatobiliary system removes the systemically absorbed adapalene.1,2
Tazarotene
Tazarotene is rapidly hydrolyzed by skin esterases into its active metabolite, tazarotenic acid. Tazarotenic acid binds mostly to RAR-beta and RAR-gamma in the epidermis and has no affinity for RXR. It modulates the expression of retinoid responsive genes to regulate cell proliferation, cell differentiation, and inflammation. Tazarotene down-regulates the expression of keratinocyte transglutaminase I (Tgase I), hyperproliferative keratins (K6 and K16), migration inhibitory factor-related protein (MRP-8), and epidermal
growth factor receptor, while inducing tazarotene inducible genes (TIGs) 1, 2, and 3.
growth factor receptor, while inducing tazarotene inducible genes (TIGs) 1, 2, and 3.
Although it is rapidly metabolized, a small amount of tazarotene can be absorbed systemically. This is degraded by oxidation to inactive sulfoxine and sulfone derivatives that are excreted in the urine and feces. In normal skin, systemic absorption can be up to 5% of the applied drug, and the maximum blood concentration of tazarotene gel is at 9 hours after the application. The half-life of the drug is less than 20 minutes, and the terminal half-life is about 18 hours.1,2
Indications
The most important aspect of treating a patient with topical retinoids is patient education regarding proper application techniques and expectations. Side effects, such as local skin irritation, and the fact that noticeable results may take months to appear should be discussed. Desquamation corresponds to the hyperproliferative response of RARs to tretinoin, although erythema does not appear to be receptor mediated. Administration of topical retinoids needs to be titrated, depending on the patient’s reaction to the medication. A general rule is to start treatment with the lowest strength formulation and then gradually increase it as the patient gains tolerance. Sunscreens and moisturizers should be incorporated as daily regimens.1
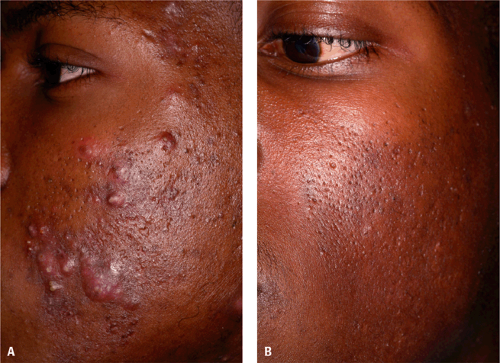 Figure 10-3 Acne treated with tazarotene 0.1% cream in an African American. A: Before. B: After. (Courtesy of Pearl E. Grimes, MD.) |
Acne vulgaris
Topical retinoids are an important, if not essential, component in the treatment of acne vulgaris (Fig. 10-3). Their mechanism of action involves its anti-inflammatory properties and its normalization of the follicular epithelium to loosen comedones and prevent sebum buildup, not sebum production. They are used for mild acne that is nonscarring, has open and closed comedones, and has moderate pustules. For moderate to severe acne, they are used in combination therapy. Topical retinoids need to be maintained to have comedolytic effects. Cystic acne is more severe and does not respond well with monotherapy topical treatment but may be useful in combination therapy or as maintenance therapy once control of the disease has been reached.2,5
In treating acne vulgaris with topical retinoids, patients must be aware that onset of improvement may take several weeks. Topical retinoids are usually applied as a thin layer of the retinoid nightly. The disease can worsen in the first month of treatment as the follicular epithelium loosens. After 2 months of sustained treatment, a continued improvement is usually noted. At this point, the main goal of treatment is to prevent development of new comedones. Combination therapy of topical retinoids with mild topical antimicrobial agents is often used.2
In a review of acne in African American patients with Fitzpatrick skin types IV through VI, adapalene




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree