div class=”ChapterContextInformation”>
1. How to Best Confirm Diagnosis Before Starting Treatment
Keywords
TrichoscopyDermoscopyAlopeciaTrichogramAnagenHairHair lossLichen planopilarisLupusAlopecia areataAndrogenetic alopeciaTelogen effluviumIn many patients presenting with hair loss, diagnosis can be made (or at least suspected) through a detailed history and clinical examination. However, in doubtful cases, some diagnostic tools such as trichoscopy and trichogram may help to confirm diagnosis and, many times, avoid invasive methods, such as a cutaneous biopsy. When a biopsy is needed, trichoscopy is also helpful in selecting the best site for the procedure. This chapter will cover the basics of trichoscopic examination, trichoscopy-guided biopsies, and the trichogram.
Trichoscopy
Dermoscopy of hair shafts and the scalp is currently regarded by many specialists as an essential part of the consultation of patients presenting with hair loss. Dermoscopy allows visualization of morphologic structures that are not readily visible by the naked eye, including perifollicular and interfollicular features, as well as, changes to hair shaft thickness and shape [1]. In 2006, the name trichoscopy was proposed for dermoscopy in the diagnosis of hair and scalp disorders, and the term is now widely adopted [2].
How to Perform Trichoscopy?
Devices
Handheld portable dermatoscopes : These devices usually only allow lower magnifications (tenfold). However, this is quite satisfactory for the daily practice, and such devices tend to be reasonably cost-effective. In addition, lower magnifications provide a better overview of a large scalp area [4].
Digital video dermatoscopes : Higher magnifications (20- to 100-fold and higher) provided by digital dermatoscopes allow better visualization of fine details, particularly of hair shaft defects and changes in scalp vessels. Another advantage of this more expensive group of devices is that they are usually equipped with photo storage and image analysis software. Cheaper video dermatoscopes that can be connected to any computer via USB are also available. These cheap devices have low image quality but still allow diagnosis in most common hair disorders [5].
Mobile-connected dermatoscopes : These are a somewhat in between and practical option which allow photography usually at a magnification of 10–20×.
The Exam
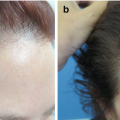
Diffuse hair loss : In this scenario, it is important to part the hair and examine at least three different sites: the frontal and middle scalp and the vertex (Fig. 1.1a). Lower magnifications (10–20×) will enable visualization of a larger area. If available, higher magnifications facilitate evaluation of hair shaft diameter, a hallmark of androgenetic alopecia (AGA). Nonandrogen-dependent areas (occipital scalp) are usually spared in AGA and can be examined for comparison (Fig. 1.1b).
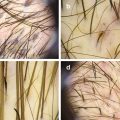
Localized hair loss : In these cases, both the affected area and the periphery of lesions should be examined. In the affected scalp, it is important to establish whether hair follicle openings are present or not. Loss of follicular openings will guide the diagnosis toward a scarring condition. Signs of disease activity may be present at the alopecic area and/or at the periphery of the lesion (Fig. 1.2), depending on the etiology. So, the periphery should always be examined, as well. In marginal alopecia, loss of vellus hairs is a typical sign of frontal fibrosing alopecia.
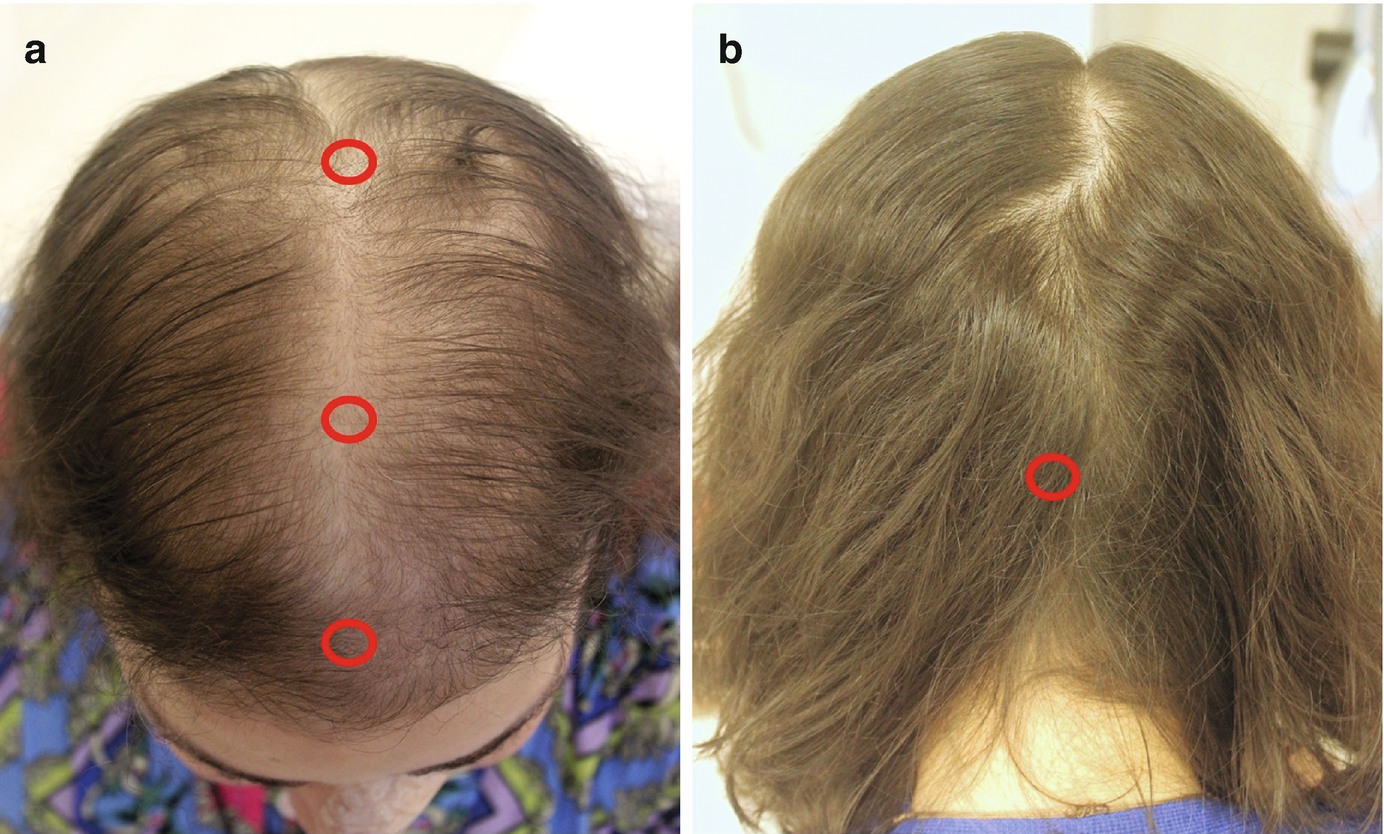
Examination sites in a patient with diffuse or patterned alopecia. (a) Frontal and mid-scalp and vertex. (b) The occipital (nonandrogen-dependent) scalp is examined for comparison
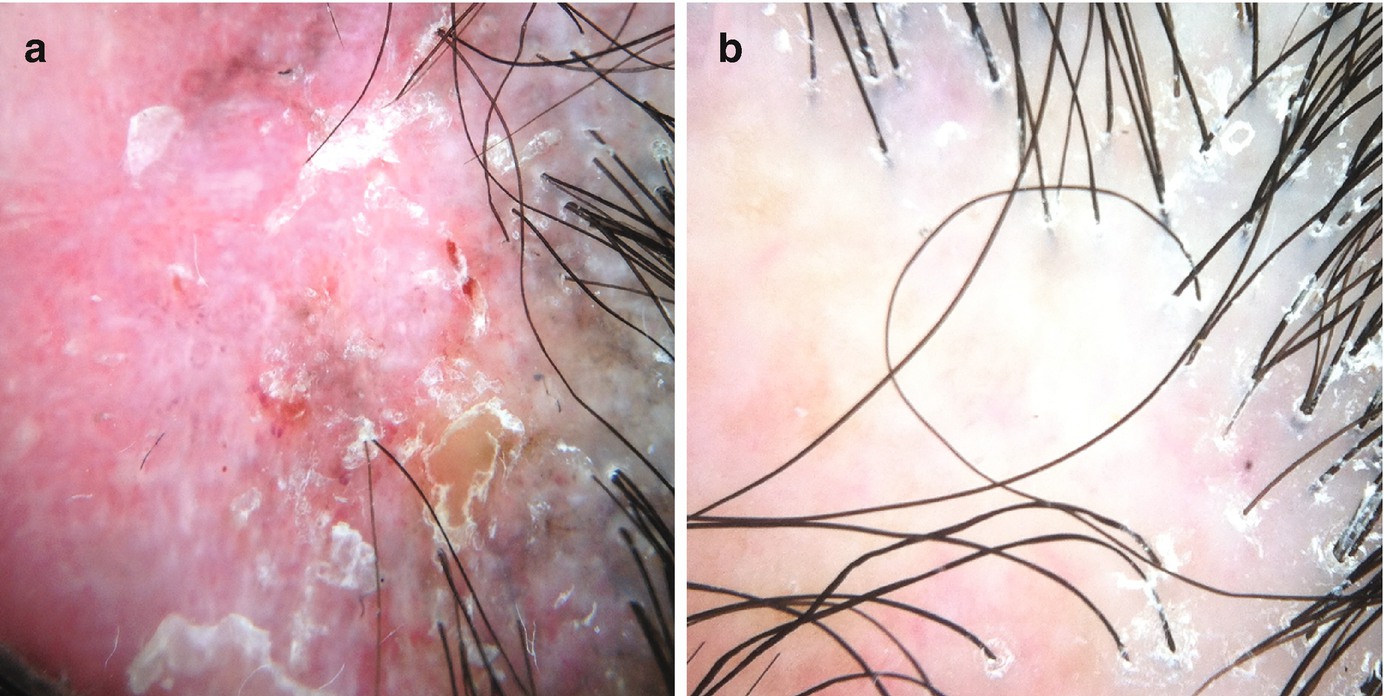
Signs of disease activity may be present at the center of the lesion and/or at its periphery. (a) Patient with discoid lupus presenting with marked erythema and scaling at the center of the lesion. (b) In this case of lichen planopilaris, the center of the lesions presents as a milky area without follicular openings, suggestive of scarring. Perifollicular scaling in the hair-bearing periphery indicated disease activity
Tips
Polarized vs. nonpolarized light : Both can be used in trichoscopy, but nonpolarized devices may require the use of an immersion fluid in order to cancel out reflections from the stratum corneum.
Immersion fluid : As a general rule, we start examination with dry dermoscopy and then use an immersion fluid if we judge necessary. Some points to consider:
Contact dermoscopy will always be necessary if an immersion fluid is being used.
Immersion fluids make the visualization of scales, vellus, and white hairs difficult (as they “disappear” when a fluid is used).
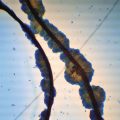
“Elimination” of scaling with immersion fluid is sometimes desirable, as excessive scaling may interfere with visualization of underlying trichoscopic features (Fig. 1.3).
Contact vs. no contact : When studying hair shafts and the scalp, contact is important; otherwise shafts will appear in different levels and out of focus. An exception is the study of vascular patterns, because excessive pressure may hamper the visualization of vessels.
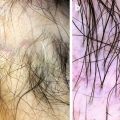
Pitfalls: Some artifacts may simulate hair disorders and lead to misdiagnosis, so it’s important to look out for them. The most important pitfalls are secondary to scalp deposits (such as dirty dots simulating black dots like the ones seen in alopecia areata, for example) (Fig. 1.4a), scalp staining (hair dye mimicking skin hyperpigmentation or, when deposited in the follicle, dots) (Fig. 1.4b), and hair shaft deposits (secondary to dry shampoos or hair styling products that may look like nits or hair casts).
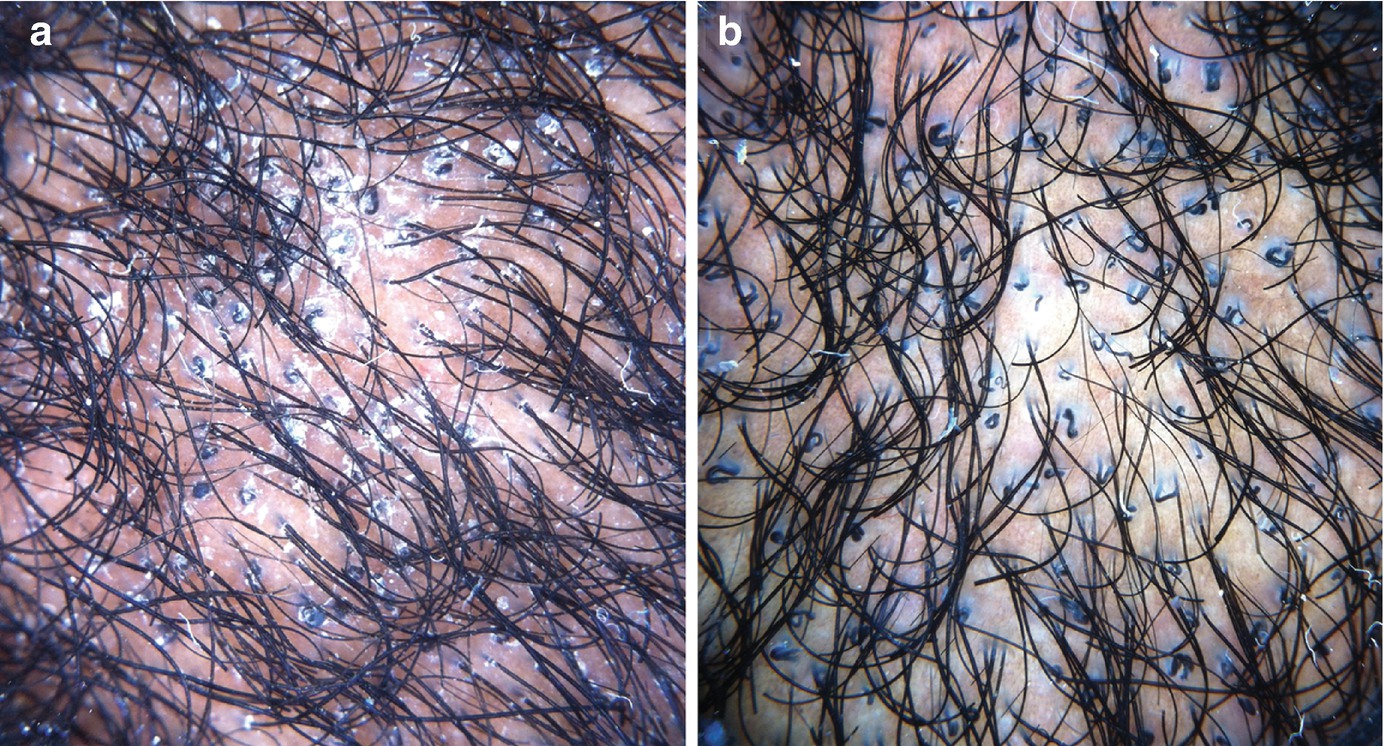
Trichoscopy of tinea capitis (a) without and (b) with the use of immersion fluid. Diagnostic features such as comma and corkscrew hairs become more visible after scales “disappear” with the use of immersion fluid
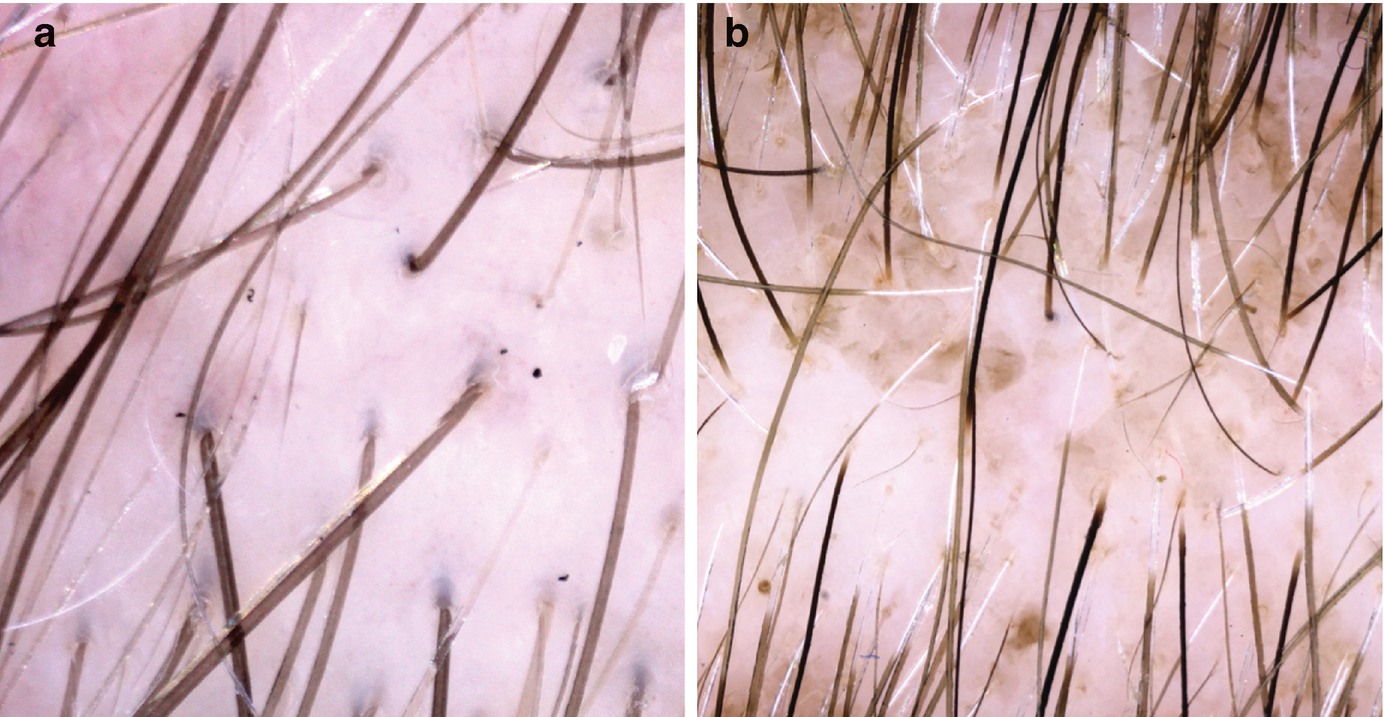
Pitfalls in trichoscopy: (a) Dirty dots, exogenous particles or fibers that may simulate trichoscopic structures; and (b) hair dye. In this case, it is staining both the interfollicular and follicular region
Trichoscopy-Guided Biopsy
The best biopsy sites in each condition
Scarring alopecias | |
Peripilar casts. If very subtle, choose a site in which peripilar casts surround small hair tufts | |
Terminal hairs surrounded by white concentric scales/peripilar casts | |
Fibrosing alopecia in a pattern distribution (Fig. 1.7) [10] | Small hair tufts with perifollicular scaling and erythema in an area with hair shaft variability |
Keratotic plugs and red dots | |
White/gray peripilar halos and/or broken hairs | |
Large hair tufts (>5 hairs) surrounded by white/yellowish scales | |
Broken hairs, large yellow dots, and keratotic plugs | |
Nonscarring alopecias | |
Acute: exclamation mark hairs, black dots, dystrophic or broken hairs. Chronic: yellow dots or circle pigtail | |
Hair shaft diameter diversity | |
Black dots, broken hairs, flame hairs | |
Hair-bearing margin showing hair casts | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree