Tissue Expansion
Ashley K. Lentz
Bruce S. Bauer
Tissue expansion provides additional cutaneous tissue, allowing the surgeon to optimize contour and color match in a given reconstructive effort. Careful planning and follow-through are necessary to achieve the desired outcome and minimize complications.
BACKGROUND
Although the genesis of modern-day tissue expansion is credited to Radovan1 and Austad,2 the technique takes some of its roots from early lessons in distraction osteogenesis. Bone traction with either internal or external devices at the turn of the 20th century paved the way for the concept that mechanical stress on tissue leads to lengthening. In the mid-1950s, Neumann3 became the first surgeon to use an expansile implant when he used a latex balloon to enlarge periauricular skin for a traumatic ear deformity. Despite these early efforts, it was not until 20 years after Neumann’s report that tissue expansion was revisited. Charles Radovan,1 a resident at Georgetown, reintroduced the concept of expansion when he inserted a contemporary device with an internally placed port. Shortly thereafter, Eric Austad2 produced a selfinflating device. In 1982, the first National Tissue Expansion Symposium was sponsored by the Plastic Surgery Educational Foundation. This marked the recognition of a new advance in reconstructive surgery. Since that time, expansion has been applied to a multitude of reconstructive problems, with applications demonstrated in both local expansion and distant expansion for subsequent graft and flap transfer. Better understanding of expansion has allowed modifications in flap design, increasing its value as a reconstructive option.4
PHYSIOLOGY
When mechanical stress is applied to skin over time, two phenomena occur: mechanical creep and biologic creep. The former is based on morphologic changes that occur on a cellular level in response to the applied stress. Mechanical creep is essentially cellular stretch. However, biologic creep is a cellular proliferation that results from the disruption of gap junctions and increased tissue surface area. Growth of the tissue by cellular proliferation restores resting tension of the stretched tissue to baseline.5 The epidermis gets thicker with concurrent thinning of the dermis and alignment of collagen fibrils. These effects are maximized at 6 to 12 weeks post-expansion. On a molecular level, various cytokines are induced in response to expansion.6
The vascularity of an expanded flap is superior to its nonexpanded counterpart in both number and caliber of vessels.7 Moreover, angiogenic factors such as vascular endothelial growth factor are expressed in expanded tissue at a significantly higher level when compared with nonexpanded controls. This augmentation in blood flow is attributable to the capsule that forms around the prosthesis. Because of the similarity between expanded and delayed flaps in vessel caliber, tissue expansion is regarded as a form of the delay phenomenon. An expanded flap, therefore, is a delayed flap.
EXPANSION DEVICES
Tissue expanders differ in size, shape, and type of filling valve. Expanders can be standard, customized to the donor site (breast), or can be designed to fill differentially to provide tapering of tissue. In terms of shape, they follow three basic patterns: round, rectangular, and crescent. The more commonly used include the round and rectangular types. The crescent-shaped prostheses were originally designed in an effort to minimize dog-ears at the donor site, but have fallen out of favor. It has been recognized that the rectangular expanders allow for additional expanded tissue, thereby increasing the possible choices for flap design (Figure 10.1).
Expander volumes have a wide range and the choice varies according to the anatomic site of expansion and need for gained tissue. Round and rectangular expanders range in size from less than 100 cc to greater than 1,000 cc in volume. Sterile technique is used to deliver saline to the valve port, which may be integrated into the expander device or attached to the expander via silicone tubing of customized length. An integrated system is favorable if only one single pocket is undermined; however, the implant may be more prone to rupture during expansion. Remote ports avoid the danger of inadvertent prosthesis rupture, but have their own set of complications including flipping or migration of the device in vivo, as well as tube obstruction. In an effort to avoid these complications, the port tunnel should be conservative in size and the port should be placed over firm supportive tissue and secured with sutures if needed.
SURGICAL PLANNING
One aspect cannot be overemphasized: The design for flap expansion should be planned prior to surgery. Consideration for the incisions, expander placement, flap movement in relation to the defect, and postoperative scars require appreciable preoperative planning. Thorough discussions with the patient and family are critical for successful reconstruction. If home tissue expansion is planned, then we suggest a separate clinic session devoted to education of the patient and family with regard to the goals of expansion, expansion technique, and the need for keen observation of the skin throughout the process.
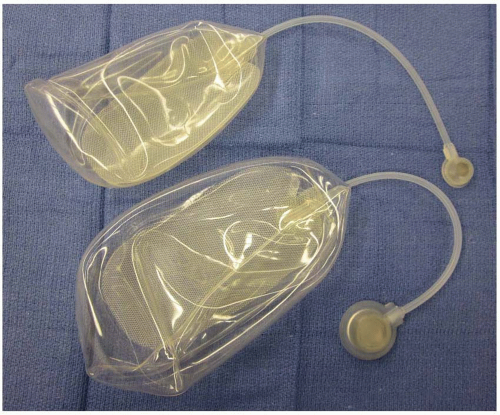 FIGURE 10.1. Rectangular tissue expanders. Size 350 and 500 mL tissue expanders with rectangular shape, thicker base plate, tubing, and a small and large remote filling port. |
Donor site choice plays an important role in expansion as the surgeon strives to provide a good match for color, texture, and contour for an optimal aesthetic and functional outcome. Infection, unstable scars, and traumatized tissue of the donor site may lead to implant failure or extrusion. When placing expanders, attention is paid to the location of the incision. If the purpose is removal of a lesion, we recommend placing the incision within the lesion borders. Gentle handling of the skin flaps is mandatory, as rough or aggressive retraction of the flaps can lead to skin edge necrosis. The port should be placed in a region of firm skeletal support, such as rib, iliac crest, or anterior thigh. Partial fill of the expander at the time of placement (approximately 10% to 20% of its listed volume) assures that the expander is properly positioned and without surface folds. Soft, flexible expanders should be used and the redundant expander should be folded underneath the expander in order to avoid future interference with the port during filling. Large expanders measuring greater than 250 mL prove more effective and we routinely use 500 mL or larger expanders. We recommend the use of larger ports for even the smaller expanders in order to avoid flipping of the port and easier palpability. Small closed suction drains are used to close the potential dead space. In most cases, the expander pocket incisions are closed in a watertight fashion with 4-0 clear Nylon sutures and 4-0 Prolene running continuous sutures. Skin flaps are dressed with Bacitracin and Xeroform gauze followed by soft 4 × 4 fluffs. Patients may or may not require overnight admission for pain control and monitoring of the skin flaps for potential compromise or hematoma formation.
Serial expansion begins 7 to 10 days post-insertion, provided that the skin flaps are in excellent condition. Drains are removed within 10 days of surgery. After detailed training and education, pediatric patients participate in a home expansion protocol directed by the parent or guardian. It has been demonstrated that home expansion is safe and equivalent to office expansion with regard to successful outcome.8 Expansion should render the skin tense, but one should not expand until it is extremely painful to the patient or cause skin compromise. Both suggest overly aggressive expansion. The home expansion protocol typically lasts 8 to 12 weeks in preparation for transfer of the expanded tissue.
Although early dogma of tissue expansion emphasized expansion as a means of generating large advancement flaps, experience demonstrates that expanded transposition and rotation flaps are frequently preferable. Clearly, the increased vascular supply of the expanded flap places little limitation on the ingenuity of the surgeon in designing flaps unique to the varied recipient defects. Although requiring more planning and forethought, transposition of the flap provides greater versatility in flap design and range.4,9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








