THORACIC RECONSTRUCTION
I. FORM AND FUNCTION
A. Protection: Vital organs of the chest
B. Flexibility: The thorax is not a static structure
C. Stability: Necessary for pulmonary function
D. Physiology—inspiration/expiration
1. Thoracic expansion = negative intrathoracic pressure, lung inflation
2. Chest wall recoil = increased intrathoracic pressure, lung deflation
II. ANATOMY AND PHYSIOLOGY
A. Skeleton
1. Posterior—12 thoracic vertebrae, bilateral scapulae
2. Anterior: Sternum, manubrium, xiphoid, and bilateral clavicles
3. Ribs
a. 10 pairs with costal cartilage, 2 pairs without
b. Superior 7 articulate directly with sternum (“true ribs”)
c. 8th, 9th, and 10th ribs (“false ribs”) have indirect articulation
d. 11th and 12th ribs have articulation only posteriorly with vertebral bodies
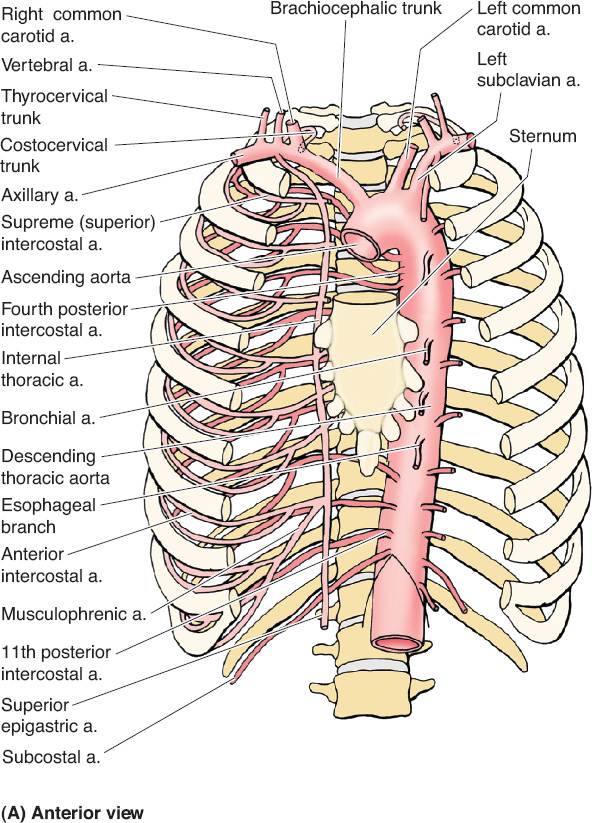
B. Vascular anatomy (Fig. 49-1)
1. Internal mammary vessels
a. Useful recipient sites for microvascular reconstruction
b. Most suitable and consistent for micro at the third intercostal space
c. Important to assess patency if planning to use rectus or pectoralis turnover flaps
d. Arises from subclavian artery near its origin travels down on approximately 1 cm from the costosternal junction
e. Runs deep to internal intercostal muscles but superficial to transverse thoracic muscles
f. Continues down until it divides into superior epigastric around the 6th intercostal
C. Musculature
1. Respiratory accessory muscles
a. Intercostals: Three layers (external, internal, and innermost)
i. Neurovascular bundle runs between internal and innermost
ii. Neurovascular bundles are inferior to rib and have vein, artery, nerve (VAN) in descending order
b. Sternocleidomastoid
c. Scalene
d. Diaphragm and muscles of the abdominal wall
2. Muscles of upper extremity movement
III. ETIOLOGY OF CHEST WALL DEFECTS
A. Infection
1. Internal
a. Empyema, fistula, and osteomyelitis
______________
*Denotes common in-service examination topics
b. Postoperative sternal/thoracotomy wounds
c. Bronchopleural fistulae
2. External
a. Abscess, soft tissue infections, cellulitis
b. Necrotizing fasciitis
3. Deep sternal wound infection/mediastinitis
a. 1% to 3% of patients after median sternotomy
b. Pairolero/Arnold classification
i. Type I: Early (days), serosanguinous drainage only
a) Treatment: Antibiotics or explore, debride, and close
ii. Type II: Early (weeks), purulent, range from cellulitis to sternal osteomyelitis
a) Treatment: Debridement and flap coverage to obliterate dead space
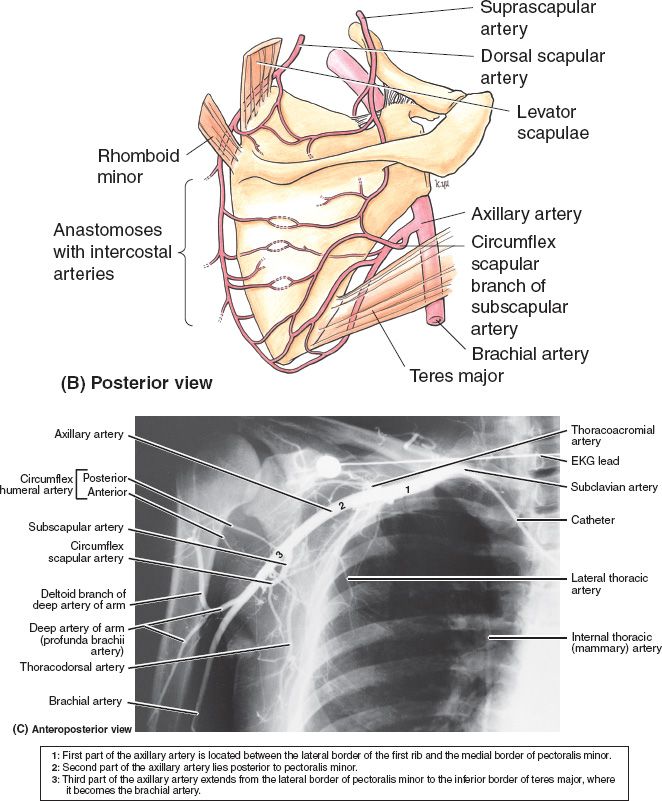
Figure 49-1. Vascular anatomy of the chest wall, axilla, and upper arm. (From Moore KL, Dalley AF, Agur AM, eds. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
iii. Type III: Late (months), chronically draining osteomyelitis
a) Treatment: Debridement and flap coverage to obliterate dead space
B. Neoplasm: Primary (i.e., sarcoma), contiguous structure (i.e., breast or pleura), or metastatic
1. Primary chest wall tumors are rare: Most commonly breast CA and sarcoma
2. Most common primary tumors are of soft tissue. Malignancy is likely.
a. #1—Malignant fibrous histiocytoma
b. #2—Rhabdomyosarcoma
3. Sternal and rib primary tumors are most commonly osteochondroma
4. 96% of sternal primary bone tumors are malignant
5. Desmoid tumors
a. Originate in muscle/fascia (40% in shoulder or chest wall areas)
b. Aggressively invasive locally
c. High recurrence rate
C. Trauma
D. Iatrogenic (i.e., radiation damage)
1. *Poland’s syndrome (sequence)
a. Pathophysiology
i. Defective development of a unilateral proximal subclavian artery (between origins of vertebral [spared] and internal mammary [compromised])
ii. Right side more common than left 2:1
iii. No difference in prevalence between male and female
b. Related conditions
i. Mobius syndrome (underdeveloped cranial nerves VI and VII)
ii. Klippel–Feil syndrome (fusion of cervical vertebrae)
c. Diagnostic findings
i. Congenital absence of the sternal head of the pectoralis major muscle
ii. Brachysyndactyly or hypoplasia of extremity
iii. Absence of costal cartilages
iv. Hypoplasia of breast and subcutaneous tissue that might affect nipple areolar complex
v. Axillary hair deficiency
d. Treatment for females
i. General goals: Achieve breast symmetry, recreate, anterior axillary fold, and provide adequate infraclavicular fullness
ii. Implant versus autogenous tissue-based reconstruction often with latissimus dorsi or combination of lat and implant
iii. Endoscopic techniques can be used to minimize incisions and improve outcomes: 6-cm incision can be made in the midaxillary line of the upper chest to perform the entire breast and chest wall reconstruction
a) Start to dissect out pocket under direct visualization
b) Insert 10-cm, 30-degree scope to improve visualization and to allow for larger pocket dissection
c) Remote port placed in separate pocket
e. Treatment for males
i. General goals: Provide a symmetric chest wall contour
ii. Latissiumus flap
iii. Custom-designed tissue expander placed through open or endoscopic techniques
2. Pectus excavatum and carinatum
a. Excavatum (“funnel chest”)
i. Chest wall concavity due to retrodisplaced sternum. Typically in lower one-half to one-third of sternum.
ii. Most common congenital chest wall deformity
iii. Male to female incidence is 4:1
iv. Indications for repair
a) Cosmetic/psychological
b) Cardiopulmonary impairment
v. Surgical options
a) Ravitch (classic approach): Cartilage resection/detachment and bar placement
b) Nuss: “Turnover” bar is placed providing retrosternal deformation pressure
c) Implant-based repair (provides fill for defect without changing intrathoracic mechanics)
d) Other/nonsurgical: Vacuum, magnetic, orthotics
b. Carinatum (“bird chest”)
i. Chest wall convexity
ii. Incidence only 20% of all pectus deformities
iii. Associated with scoliosis, familial pectus deformity (any), mitral valve prolapse, and osteogenic syndromes
iv. Surgical options
a) Ravitch; reverse Nuss procedure; orthotics/bracing
IV. TECHNICAL ASPECTS OF RECONSTRUCTION AND MATERIALS
A. Skeletal defects
1. *Indications for skeletal repair
a. Skeletal injury/resection >5 cm in diameter
b. Greater than 4 to 5 ribs with potential flail chest
c. Goal: Avoid flail chest, restore protective structure, maintain physiologic function
d. Defects <5 cm diameter usually recover well without rigid reconstruction
2. Autologous reconstructive options
a. Bone grafting
i. Potential donor sites: Split rib, iliac crest, and fibula
ii. Bone graft should be aligned with vascularized trabecular bone to facilitate osteoconduction
b. Fascia relocation (i.e., fascia lata graft)
i. Only a semi-rigid substitute for bone
ii. Drawbacks: Flaccidity, prone to infection, poor protective barrier
3. Prosthetic materials
a. Methyl methacrylate (poly-MMA): Exothermic polymer used as a bone cement, nonbiologic with multiple potential complications possible including embolization, burns, difficulty fixing to bone, infection, or erosion
b. Gore-Tex, Marlex, Vicryl, Teflon, Prolene available mesh materials
c. Marlex “sandwich” used in composite reconstruction with methyl methacrylate
B. Soft tissue defects and coverage
1. Skin grafting
a. Considerations: Must have viable blood supply at base, will not fill dead-space or cavities, poor candidate in irradiated areas
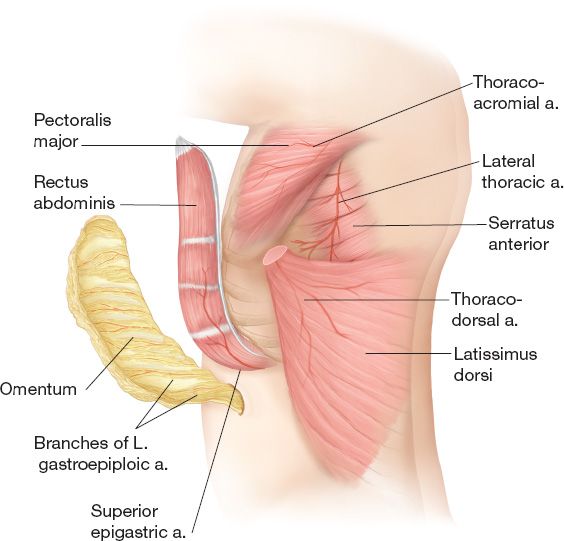
2. Local reconstructive options (Fig. 49-2) (See also Chapter 4—Table 3)
a. The goal for thoracic reconstruction is complete debridement followed by closure with well-vascularized tissue (typically muscle)
b. For intrathoracic defects, dead space obliteration may also be required
c. *Pectoralis major flap is the workhorse
i. Mathes–Nahai type V
ii. Dominant: Thoracoacromial vessels (regional artery: subclavian)
iii. Minor: Internal mammary perforators, anteromedial/anterolateral intercostal perforators
iv. Considerations: Internal mammaries must be present to divide thoracoacromial (commonly used in cardiac procedures)
d. Latissumus dorsi flap (Mathes–Nahai type V)
i. Useful in reconstruction of anterior and posterior defects
ii. Dominant: Thoracodorsal vessels (regional artery: subscapular)
iii. Minor: Posterior intercostal perforators, retrograde flow from the artery serratus (terminal branch of thoracodorsal artery)
iv. Considerations: High rate of seroma, possible functional disability
e. Rectus abdominis flap (Mathes–Nahai type III)
i. Both pedicled and free flap options possible
ii. Commonly deep inferior epigastric artery perforator flap (DIEP), superficial inferior epigastric artery flap, TRAM, free transverse rectus abdominis musculocutaneous flap (TRAM), vertical rectus abdominis musculocutaneous flap (VRAM)
iii. Major pedicles
a) Superior epigastric off of the internal mammary
b) Inferior epigastric off of the external iliac
iv. Deep inferior, superficial inferior, and superior epigastric vessels contribute
v. Considerations
a) High donor site morbidity and complications
Figure 49-2. Common pedicled flaps for thoracic or abdominal wall reconstruction and their arterial anatomy.
b) Can harvest as a pedicled VRAM if IMA on that side has been used for CABG, by basing blood supply on the highest intercostal to the rectus muscle.
f. Trapezius flap (Mathes–Nahai type II)
i. Muscle or musculocutaneous, can reconstruct superior aspect of chest wall with pivot at posterior base of the neck
ii. Dominant: Transverse cervical vessels (off thyrocervical trunk (80%) or subclavian artery (20%)
iii. Minor: Posterior intercostal perforators and occipital vessels
g. Parascapular flap
i. Useful in reconstruction of shoulder, axilla, and lateral chest
ii. Dominant: Circumflex scapular a. (with venae comitantes)
iii. Pivot point at vascular pedicle in the triangular space
h. Serratus anterior flap (Mathes–Nahai type III)
i. Can be used as muscle, musculocutaneous, or fascial flap
ii. Cannot take entire muscle or winged scapula will result
iii. Dominant: Lateral thoracic a. and thoracodorsala.
iv. Consideration: Useful in filling intrathoracic cavities
i. External oblique flap (Mathes–Nahai type IV)
i. Posterior intercostal vessels
ii. Useful in lower one-third chest wall defects in which the latissimus m./rectus may be compromised by previous radiation or surgery
j. Omental flap (Mathes–Nahai type III)
i. Dominant: Right and left gastroepiploic vessels
ii. Pivot on right: First portion of duodenum
iii. Pivot on left: Splenocolic ligament
iv. Tunnel necessary through costal margin or diaphragm usually
v. Can be harvested laproscopically or with a laparotomy
k. Other local trunk/back flaps: Paraspinous muscle flap (type IV)
3. Free flap considerations
a. Aids in decision-making tree when vascular supply may be compromised
b. Increased freedom in flap positioning
c. Most common: Rectus abdominis free flap (DIEP or free TRAM)
d. Other: Gluteus maximus (wounds of the lower back/trunk)
ABDOMINAL RECONSTRUCTION
I. FORM AND FUNCTION
A. Protection: Vital organs of the abdomen
B. Forces: The abdomen is under cylindrical forces with the inward abdominal wall and diaphragm pressures equilibrating with outward intraperitoneal pressures
C. Physiology: Abdominal strength derived from tendinous connections and soft tissue
1. Valsalva: Used to brace body and increase intraperitoneal pressure
2. Herniation: Results in pressure outlet and inability to effectively control abdominal pressure with Valsalva forces
D. Goals of reconstruction
1. Protection for the abdominal viscera
2. Prevention of fluid losses
3. Fascial support
4. Aesthetics
5. Hernia repair: Restore normal abdominal counter-pressure forces and cylinder continuity; thus restoring proper muscle function and trunk mechanics
II. ANATOMY AND PHYSIOLOGY
A. Skeleton
1. Lower thoracic ribs (superiorly to costal margin; posteriorly to costovertebral angle)
2. Lower thoracic spine and lumbar spine (posteriorly)
3. Pelvis (inferiorly)
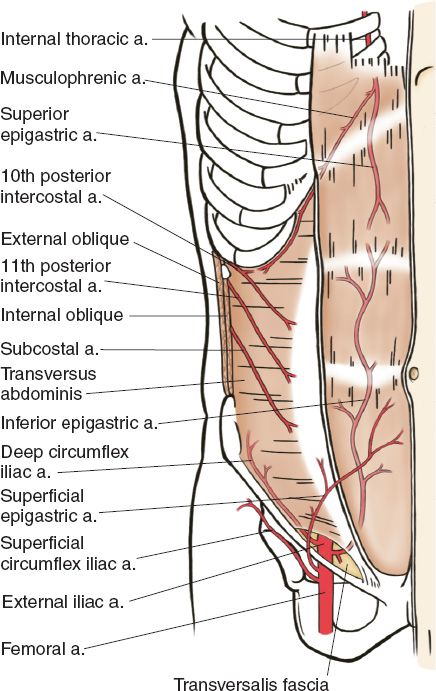
B. Arterial supply of the abdominal wall (Fig. 49-3; also see “Body Contouring” chapter)
C. The anterior abdominal wall
1. Eight layers lateral to semilunar line
a. Skin
b. Subcutaneous tissue/Camper fascia
c. Scarpa fascia
d. External oblique muscle
e. Internal oblique muscle
f. Transversus abdominis muscle
g. Transversalis fascia
h. Peritoneum
2. Central abdomen
a. Paired rectus muscles
b. Linea alba
3. *The rectus sheath and the arcuate line of Douglas
a. Rectus sheath above the arcuate line
i. Anterior: External oblique and the anterior leaf of the internal oblique aponeuroses
ii. Posterior: Posterior leaf of the internal oblique aponeurosis, transversus abdominis, and transversalis fascia
b. Rectus sheath below the arcuate line
i. Anterior: External and internal oblique aponeuroses and the transversus abdominis muscle
ii. Posterior: Transversalis fascia
Figure 49-3. Arterial anatomy of the hemiabdominal wall (From Moore KL, Dalley AF, Agur AM, eds. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
III. ETIOLOGY OF ABDOMINAL WALL DEFECTS
A. Surgical dehiscence
1. A separation of the layers of a wound. May be partial, superficial, or complete with separation of all layers and full interruption. Complete dehiscence of an abdominal wound may lead to evisceration (see below).
2. Risk factors: Increased age, obesity, genetic predisposition, diabetes, infection, fluid collection, postoperative trauma, or technical error will all increase risk
3. Planning for abdominal reconstruction
a. Nutrition status
b. Inflammation/infection
c. Exposed viscera (is exposed bowel “frozen” or free)
4. Evisceration
a. Pristine wounds may be closed primarily (rare)
b. Principles
i. Delay definitive closure until wound decontaminated
ii. Avoid wet-dry or other debriding dressings on bowel
iii. Vacuum assist devices effective for swelling (not used in grossly contaminated, infected, or malignant wounds)
c. Large wound with exposed viscera may need to be closed with skin grafting directly on bowel if long delays to closure occur; can be performed after granulation tissue present on bowel
d. Fistula management: Tube drainage, grafting, closure after 3 to 6 months
B. Congenital
1. Gastroschisis
a. Midline umbilical defect without soft tissue coverage
b. Failure of midline mesoderm embryologically
2. Omphalocele: Periumbilical defect with sac developing exposed abdominal viscera
3. Congenital umbilical/inguinal hernias
a. Most pediatric surgeons recommend repair of inguinal hernia
b. Delay repair of congenital umbilical hernias until 4 to 5 years of age with conservative observation, as many resolve spontaneously
4. Beckwith–Weidman syndrome
a. Sporadic syndrome related to mutations on chromosome 11, characterized by increased tissue growth (large features—tongue, gigantism, hemihypertrophy), increased cancer risk, and midline abdominal defects
b. Diastasis recti, umbilical hernia, or omphalocele may occur
c. Additional findings: Ear pits, nevus flammeus, neonatal hypoglycemia
IV. TECHNICAL ASPECTS OF SOFT TISSUE RECONSTRUCTION AND MATERIALS
A. Diastasis recti
1. Spreading of the linea alba resulting in of parallel rectus muscles
2. Do not confuse with a ventral hernia. There is no palpable fascial edge with rectus diastasis.
3. May be amenable to physical therapy, fascial plication
B. Midline abdominal herniation
1. Direct repair: Reserved for defects <3 cm or with easily opposable edges
2. Hernias >3 cm may be amenable to laparoscopic versus open repair with mesh. Allow additional 3 cm of overlap of mesh and posterior rectus sheath.
3. Alloplastic mesh
a. Polypropylene (Prolene, Marlex): Highly porous, good incorporation; common. May be paired with polytetrafluoroethylene (PTFE), with Gore-Tex layer on bowel contact surface
b. PTFE (Gore-Tex, polytetrafluoroethylene): Smooth, nonporous; lower foreign-body reaction, low bowel adherence, and low fistula rates. Low adhesion/incorporation to body wall.
c. Polyester (Mersilene)
d. Polygalactin (Vicryl): Satisfactory in contaminated wounds, but only as a temporary solution, since it is biodegradable
4. Biologic mesh and materials*
a. Human dermis (Alloderm, Allomax, FlexHD): Differences in sterilization techniques; very few indications to use in abdominal ventral hernia repair due to high recurrence and infection rates may be placed intraperitoneally or as adjunct to alloplastic repair or separation of parts
b. Porcine dermis (Permacol, Strattice, Collamend, Xenmatrix: May be a good alternative in contaminated fields, with low overall recurrence rates (0% to 15%) variable cross-linkage (i.e., Permacol with chemically cross-linked diisocyanate bonds)
c. Porcine intestinal submucosa (Surgisis, Fortagen): May have higher recurrence rates in contaminated fields (30% to 39%); while performing better in clean fields (0% to 5.3%)
5. Autogenous materials
a. Tensor fascial lata
i. Mathes–Nahai Type I
ii. Dominant: Ascending branch of lateral femoral circumflex artery
iii. May need delayed procedure to improve flap tip viability; can be rotated from the leg into the surgical defect
iv. Can be used as a free, nonvascularized graft: Historically well reported for abdominal wall repair
a) Sizes up to 22 × 12 cm
b) Long-term recurrence rate (∼30%)
c) Donor-site morbidity high (∼50%)
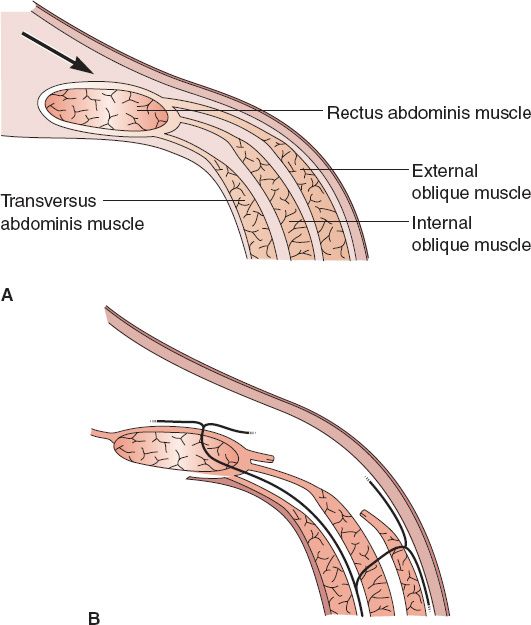
b. *Separation of parts (SOP, also referred to as “component release” or “component separation”; Fig. 49-4)
i. Technique of component separation of the abdominal wall
a) Removal of scarred, thin midline tissues
b) Division of external oblique muscle/fascia from costal margin to near inguinal ligament from semilunar line
c) Blunt separation of external oblique from internal oblique to allow for movement medially
d) Cut posterior rectus sheath uni- versus bilaterally if additional length is needed
e) Bilateral SOP can allow closure of 10cm/20cm/6cm in superior/middle/inferior thirds of abdominal wall, respectively
f) If open approach, preservation of periumbilical perforators decreases skin loss
Figure 49-4. Separation of parts (component separation) technique. A: The anterior rectus sheath is often accessed through a vertical midline abdominal incision. B: The external oblique fascia is divided at the semilunar line. Note that the nerve supply to the abdominal wall musculature is preserved. (From Mulholland MW, ed. Greenfield’s Surgery. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.)
g) Can use endoscopic techniques improve visualization and decrease dissection
ii. Preserves cutaneous vascular supply
iii. Can be augmented with the use of mesh underlay
c. Panniculectomy: Role in repair of infraumbilical hernia in obese patients
PEARLS
1. Extensive debridement and removal of foreign bodies are critical to preparing wounds for reconstruction
2. If a chest wound is greater than 5 cm in diameter or there are greater than four missing ribs, skeletal reconstruction is necessary
3. Locoregional muscle flaps are the mainstay for thoracic reconstruction using pectoralis major and rectus abdominis flaps
4. Separation of parts can gain 10, 20, and 6 cm of advancement in the superior, middle, and inferior thirds of the abdominal wall, respectively
5. Understand healing aspects and applications of biologic and synthetic mesh
QUESTIONS YOU WILL BE ASKED
1. Mathes–Nahai flap classification of flap types (know dominant and minor vessels, see Chapter 4: Flaps).
2. What layers of the abdomen are separated in a ventral hernia repair using separation of parts technique?
The External and internal oblique laterally; posterior rectus sheath from rectus muscle medially.
3. What is blood supply to abdomen?
Mid-abdomen: deep epigastric arcade (zone I);/lower abdomen: external iliac artery (zone II); and the lateral abdomen: intercostal, subcostal, and lumbar arteries (zone III).
4. How large of a hernia defect can be closed with a separation of parts closure?
Unilateral: 5 cm in the epigastric region, 10 cm at the umbilicus, and 3 cm in the suprapubic region.
THINGS TO DRAW
1. Draw the thoracic vascular anatomy in detail including anterior chest, axilla, and scapular anastamoses (Fig. 49-1).
2. Draw the abdominal vascular anatomy including intercostals, epigastric vessels, and superficial circumflex femoral vessels Fig. 49-2.
3. Draw the procedural basics for an abdominal component separation Fig. 49-4.
Recommended Readings
Pairolero PC, Arnold PG. Management of infected median sternotomy wounds. Ann Thorac Surg. 1986;42(1):1–2. PMID: 3729602.
Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–526. PMID: 2143588.
Shestak KC, Edington HJ, Johnson RR. The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg. 2000;105(2):731–738; quiz 739. PMID: 10697187.
Smart NJ, Marshall M, Daniels IR. Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon. 2012;10(3):159–171. PMID: 22436406.
< div class='tao-gold-member'>