Name
Company
Source tissue
Alpha-gal removed
DermaMatrix
MTF (Synthes)
Human dermis
NA
Flex HD
MTF (Ethicon)
Human dermis
NA
Neoform/AlloMax
Tutogen (Mentor)
Human dermis
NA
AlloDerm
LifeCell
Human dermis
NA
Strattice
LifeCell
Porcine dermis
Yes
SurgiMend
TEI Biosciences
Fetal bovine dermis
No
Veritas
Synovis
Bovine pericardium
No
The goal of using regenerative tissue matrices in reconstructive surgery is to establish an environment that enables the patient to “regenerate” tissue other than scar or foreign body capsule that mimics the autologous tissue and allows the surgeon to achieve an excellent outcome with durable esthetics and function.
23.3 Biologic Matrix Applications in Breast Reconstruction
Reconstructive options for using biologic matrices in breast reconstruction include the following:
Implant reconstruction
Expander reconstruction
Augmentation of the reconstructed nipple
Abdominal wall reinforcement
Reducing capsular contracture after radiation therapy.
The aim of this chapter is to discuss the use of acellular dermal matrices in implant and expander reconstruction.
23.3.1 Implant Reconstruction
Patients undergoing skin-sparing mastectomy for breast cancer may be candidates for either immediate implant or expander insertion. Direct-to-implant insertion is becoming an increasingly attractive proposition as methods to assess skin viability become more available. Prerequisites for successful direct-to-implant insertion include a well-vascularized skin envelope and adequate skin surface area. The use of indocyanine green based fluorescence imaging has revolutionized our ability to assess skin vascularity at the time of mastectomy. If the skin envelope is viable, an implant of size similar to that of the original breast volume may be inserted without fear of postoperative necrosis. Unfortunately, such implant placement requires accuracy of implant positioning and maintenance of that position if the esthetic outcome is to be acceptable to both the patient and the surgeon. The mastectomy pocket is, by definition, larger than the space occupied by the implant. There is a tendency for the implant to fall laterally and inferiorly as well as to slide out from beneath the pectoralis major muscle into a subcutaneous plane. To correct both of these issues, a sheet of acellular dermal matrix can be used to reduce both pectoralis major muscle window shading and control the implant pocket dimensions and location [1]. The larger the implant and the greater the degree of ptosis required, the larger this sheet of matrix should be. My personal preference is for a sheet of 8 × 16 cm for most expander reconstructions, and an additional 6 × 16 cm sheet may be necessary for large (700–800 cm3) implant reconstructions. In addition, the surgeon can use AlloDerm as a lower pole reinforcement to reduce both lower pole implant rippling and long-term capsular contracture.
23.3.1.1 Operative Technique
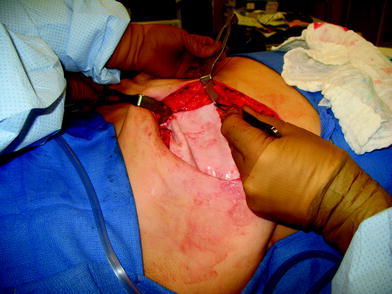
The perfusion and viability of the mastectomy skin envelope should be carefully assessed prior to committing to a direct-to-implant approach. It is the author’s preference to use indocyanine green laser fluorescence for this assessment as it is quick, easy, and exceptionally accurate. The inferolateral border of pectoralis major muscle is grasped with an Alice tissue forceps (Fig. 23.1) and the subpectoral plane is entered (Fig. 23.2). Pectoralis major muscle is released from the 6 o’clock to 3 o’clock position on the right and from the 6 to 9 o’clock position on the left (Fig. 23.2a), producing a release that gives rise to the window shade effect of the muscle. A sheet of AlloDerm or Strattice (LifeCell, Branchburg, NJ, USA) is washed in saline for 2 min to rinse off preservatives (Fig. 23.3). The superomedial corner of the matrix is sutured to the inferomedial cut edge of the pectoralis major muscle with running 2-0 polydioxanone suture (Fig. 23.4). The suture is run along the medial breast border (Fig. 23.5), then across the curve of the inframammary crease and can be sutured to a raised cuff of serratus anterior fascia laterally which provides an additional domain for an implant if required. This creates an inferior sling of AlloDerm into which an implant or expander can be placed (Fig. 23.6). The device is placed beneath the AlloDerm inferiorly and the Strattice superiorly, following which the caudal edge of the pectoralis major muscle is sewn to the cephalad edge of the AlloDerm with running 2-0 polydioxanone suture (Fig. 23.7). This creates complete coverage of the implant with the mesh. It is essential that a drain be placed between the AlloDerm and the overlying skin in order to minimize seroma formation, which could inhibit contact between the mesh and the skin, thereby reducing vascular ingrowth and incorporation. The skin is then closed with absorbable subcutaneous and subcuticular sutures in a two-layer closure sealed with cyanoacrylate cement, SteriStrips, and an occlusive water-proof dressing such as Tegaderm (Fig. 23.8).
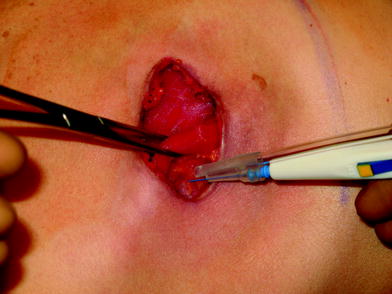
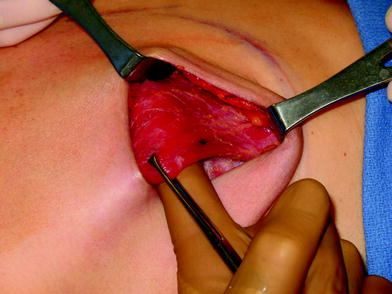
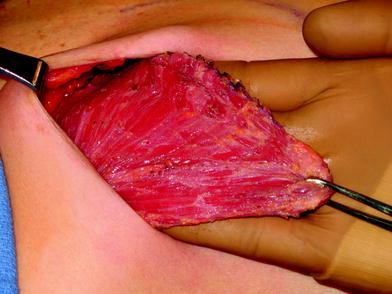
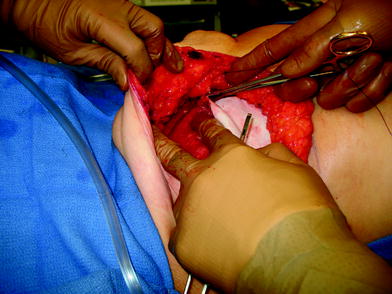

Fig. 23.1
The inferolateral border of pectoralis major is elevated with cautery

Fig. 23.2
The subpectoral plane is elevated

Fig. 23.3
The pectoralis major muscle is elevated after incising the origin inferomedially

Fig. 23.4
The sheet of acellular dermal matrix is sutured t the cut origin of pectoralis major medially