37 The Urinary Tract in Pregnancy
ANATOMY OF THE URINARY TRACT IN PREGNANCY
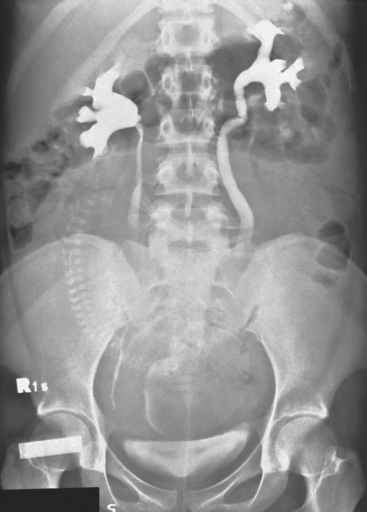
The most striking anatomic change in the urinary tract is dilation of the ureters (Fig. 37-1). Bilateral dilation of the calyces, renal pelvis, and ureters can be seen early in the first trimester and is present in 90% of women in the late third trimester or early puerperium. The changes are usually more prominent on the right and may persist for 3 to 4 months. In 11% of women, ureteral dilation persists indefinitely. In addition, there is reduced ureteral peristalsis and a greater volume of residual urine compared with the nonpregnant state. It is not known whether these patients suffer adverse sequelae, such as persistent asymptomatic bacteriuria from persistent ureteral dilation.
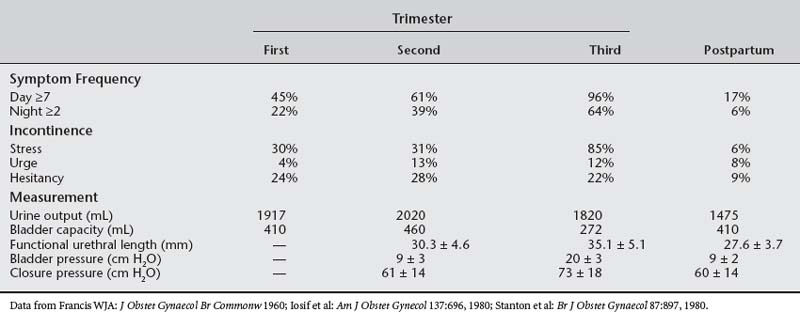
The capacity of the urinary tract increases during pregnancy. Bladder volume during pregnancy increases to 450 to 650 mL, compared with 400 mL in nonpregnant controls (Table 37-1). The hydronephrotic ureters can hold as much as 200 mL extra urine; however, no changes appear in the contraction patterns on retrograde cystometry. Depending on maternal position, uterine size, and position of the fetus, the functional volume of the bladder and ureters is dynamic in the third trimester. This increased functional volume, coupled with high urine flows (especially with fluid mobilization at night), causes polyuria and nocturia in most pregnant women.
In the past, the elevated progesterone levels that accompany pregnancy were thought to cause smooth muscle relaxation and subsequent hypotonicity and hypomotility of the ureter, defects that would contribute to ureteral dilation. Contrary to the latter observation, the large doses of synthetic progesterone used in cancer chemotherapy do not cause ureteral dilation. Measurements of ureteral tone during pregnancy reveal an increase in ureteral tone and no decrease in frequency or amplitude of ureteral contractions. Histologic study of the ureters of pregnant animals reveals smooth muscle hypertrophy and hyperplasia of the connective tissue. Thus, progesterone probably plays a small role in ureteric dilation during pregnancy.
RENAL CHANGES IN PREGNANCY
Much of the data relating to the effect of pregnancy on renal hemodynamics are derived from small studies in which measurements show considerable individual variation. Autoregulation maintains renal blood flow at a relatively constant level despite wide variations in perfusion pressure (mean renal artery pressure). Renal blood flow is usually assessed by ρ-aminohippurate clearance, which measures effective renal plasma flow (ERPF). The ERPF significantly increases during pregnancy. It reaches a peak increment in midtrimester of 50% to 85% and then shows a small decline during the third trimester that is unrelated to posture. The ERPF and GFR in pregnancy are markedly affected by posture, being maximal when the pregnant woman lies on her side. Normal pregnancy is associated with plasma volume expansion and an increase in the GFR of 40% to 65% (measured by insulin clearance) and a decrease in GFR of approximately 15% to 20% late in the third trimester. The mechanisms responsible for the increase in GFR, plasma volume, and renal plasma flow rate are unknown. Nitric oxide, endothelin, and relaxin may play a role in renal vasodilation in human pregnancy. Changes in renal anatomy, hemodynamics, and tubular function are listed in Box 37-1.
TESTS OF RENAL FUNCTION IN PREGNANCY
Tests of renal function in pregnancy must be interpreted in relation to the changes in plasma volume, glomerular filtration, and tubular reabsorption that normally occur with advancing gestation. Many of the commonly used tests of function yield lower results in pregnancy than in the nonpregnant state. Consequently, values that may be regarded as normal in the nonpregnant state may well indicate renal dysfunction in pregnancy.
The 24-hour creatinine clearance is the best clinical measurement of GFR. By week 8 of pregnancy, the creatinine clearance rate normally increases by 45% and remains elevated during the second trimester. In the final weeks of pregnancy, creatinine clearance usually declines to near nonpregnant levels (Fig. 37-2).
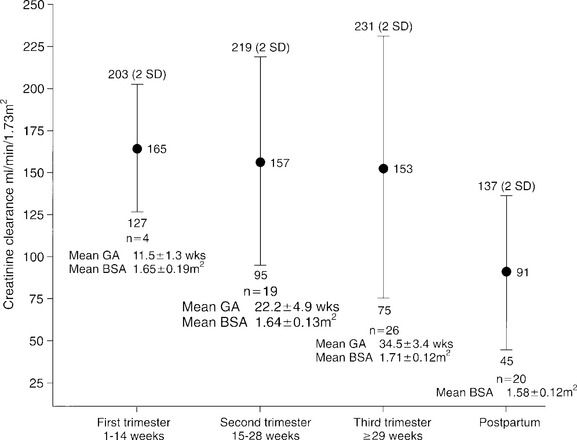
Figure 37-2 Changes in creatinine clearance during pregnancy, by trimester. BSA, Body surface area; GA, gestational age.
Urinalysis is essentially unchanged during pregnancy. However, many variables can affect the results. Normal kidneys should be able to concentrate urine to a specific gravity of 1.026 or more and to dilute urine to a value less than 1.005. In pregnancy, posture affects urine concentration and specific gravity. Urine tends to be more dilute after a left lateral position is maintained compared with an upright position. The urine must be at room temperature for the dipsticks to be reliable. Dipsticks exposed to air will give false-positive results for glucose and false-negative results for blood. Observational error and training also affect the sensitivity of predicting proteinuria by dipstick. Saudan et al. (1997) showed that the use of an automated urinalysis device for the detection of proteinuria reduced the false-positive rate. Proteinuria diagnosed on dipstick should be confirmed with a 24-hour urine collection.
Laboratory investigation begins with urinalysis of protein, glucose, ketones, specific gravity, and sediment. Glucosuria is usually detected with a glucose oxidase–impregnated dipstick. In pregnancy, the most common reasons for persistent glucose in the urine are physiologic glucosuria of pregnancy and diabetes. However, the possibility of primary renal disease with renal glucosuria should be considered. Conventional screening for proteinuria uses a dipstick that is sensitive to albumin. Five percent of healthy adults exhibit postural proteinuria, a benign condition; this can be ruled out by comparing protein levels in the first voided urine with a specimen obtained after the woman was upright for several hours. False-positive results for protein can be due to concentrated urine, many white blood cells in the urine, or vaginal secretions with epithelial cells. Fever, stress, and exercise can also cause transient proteinuria.
Proteinuria in pregnancy should be evaluated using a 24-hour urine collection and should not be considered pathologic until it exceeds 300 to 500 mg in 24 hours. Higby et al. (1994) showed that the upper limit or normal was 260 mg for urinary protein and 29 mg for albumin in a 24-hour period. Both increased after 20 weeks’ gestation. Studies have shown that a 2-hour or 12-hour collection of urine correlates with creatinine clearance and protein measured in a 24-hour specimen. Evans and associates calculated total protein using a protein/creatinine ratio in the 2-hour group and compared the results with the 24-hour urine (1840.8 ± 786 and 1944 ± 1060 mg [mean ± SE], respectively, r2 = 0.95, p < .0001). The nephritic syndrome is characterized by greater than 3 to 3.5 g/24 hours. In assessing the significance of proteinuria in pregnancy, the clinician should remember that increasing protein excretion with advancing gestation associated with known renal disease does not necessarily indicate significant progression of the disease.
URINARY TRACT DISEASES IN PREGNANCY
Urinary Tract Infection
PATHOPHYSIOLOGY
Acute pyelonephritis is a common complication in women and is responsible for a large number of office consultations with physicians and a large number of hospital admissions every year in the United States. In general, most nonpregnant women with pyelonephritis are treated in ambulatory settings. Risk factors for pyelonephritis in these women include a family history of UTI, diabetes mellitus, and the presence of urinary incontinence.
The physiologic changes of pregnancy increase the likelihood of symptomatic upper urinary tract disease, resulting in maternal and fetal morbidity and, occasionally, mortality. In fact, UTIs are one of the most common medical complications of pregnancy (Table 37-2). Thus, an understanding of the pathogenesis, clinical presentation, diagnosis, therapy, and prognosis is essential.
Table 37-2 Incidence of Urinary Tract Infection During Pregnancy
| Infection | Incidence (%) |
|---|---|
| Asymptomatic bacteriuria | 2–11 |
| Acute cystitis | 1–4 |
| Acute pyelonephritis | 1–2 |
DIAGNOSIS
The diagnosis of urinary tract infection has been based on the landmark studies by Kass (1956) and Elder et al. (1971) at Boston City Hospital. In a population of asymptomatic pregnant and nonpregnant women, a bacterial colony count of ≥105 colony-forming units (cfu) per milliliter in two or more clean-catch midstream urine (MSU) specimens reliably distinguished infection from contamination. Between 10% and 20% of pregnant women with an initial positive culture have a second negative culture within a week, even without antibiotic therapy. Furthermore, 95% of patients with clinical pyelonephritis have persistent positive cultures at ≥105 cfu/mL. Since these studies, most clinicians have used the criterion of 105 cfu/mL on a clean-catch MSU specimen to diagnose UTI. However, this criterion has not proved to be sufficiently predictive in nonpregnant women with acute dysuria or infections with fastidious organisms, or in catheterized patients.
The classic criterion of ≥105 cfu/mL for diagnosing UTI is challenged in catheterized women. Stark and Maki (1984) demonstrated that 96% of patients with low levels of coliform bacteriuria (<105 cfu/mL) progressed to ≥105 cfu/mL within 3 days if they did not receive antibiotics and remained catheterized. Although these observations help with the interpretation and management of positive urine cultures in catheterized patients, the questions of who and when to sample the urine in catheterized patients remain unanswered.
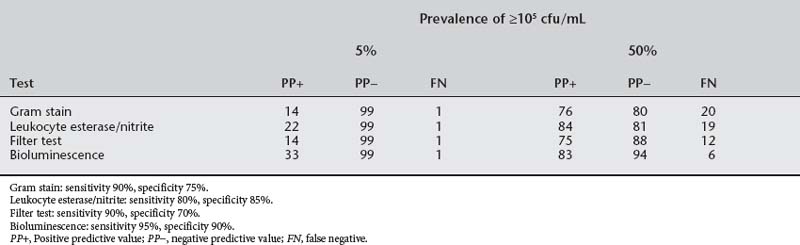
The essential question is whether culture-independent tests are sufficiently robust to replace or enhance urine culture in the diagnosis of urinary tract infection. Table 37-3 depicts the efficiency of culture-independent tests at common prevalences of urinary infection at ≥105 cfu/mL: 5% to reflect the prevalence of asymptomatic bacteriuria in pregnancy and 50% to reflect the prevalence of positive culture in women with dysuria. A false-negative rate of 6% to 20% with the culture-independent tests does not qualify them to supplant urine culture; however, many clinicians recommend that acutely dysuric nonpregnant women be treated without a culture. In pregnancy, the acutely dysuric women should always have a culture because 10% to 15% of positive cultures have group B streptococcus, an organism that predicts adverse pregnancy outcome.
At a prevalence of 2% to 10%, as is seen in asymptomatic bacteriuria during pregnancy, the positive predictive values of culture-independent tests drop precipitously in both theory (see Table 37-3) and practice and should not be used for diagnosis. On the other hand, the negative predictive value is 98% or more with any of these tests. In a low-risk population, urine testing for leukocyte esterase and nitrite on a clean-catch, first-void midstream specimen can supplant urine culture. In high-risk groups (Box 37-2), a culture should be obtained each trimester.
ASYMPTOMATIC BACTERIURIA
The microbiology of urinary tract infections in pregnancy is summarized in Table 37-4. The predominant organism is E. coli, and the identification markers and virulence traits from strains isolated from pregnant women with pyelonephritis do not differ significantly from those found in strains isolated from nonpregnant women with pyelonephritis. The pyelonephritic E. coli strains and strains from asymptomatic bacteriuria or cystitis patients differed in resistance to serum antibodies (83% vs 51%, P < .05) and epithelial adherence (63% vs 19%, P < .001).
Table 37-4 Microbiology of Urinary Tract Infections in Pregnancy
| Organism | Percentage |
|---|---|
| Escherichia coli | 60–80 |
| Klebsiella pneumoniae-Enterobacter | 3–5 |
| Proteus sp. | 1–5 |
| Streptococcus faecalis | 1–4 |
| Group B streptococcus | 4–8 |
| Staphylococcus saprophyticus | 1–3 |
Uncomplicated, asymptomatic bacteriuria is a significant health risk for pregnant but not nonpregnant women. Asymptomatic bacteriuria has been associated with pyelonephritis, preterm birth, hypertension, and fetal neuropathology. The most consistent association is a greater likelihood of pyelonephritis. In 1699 patients with untreated asymptomatic bacteriuria (Sweet [1977
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree