The Surgical Management of Locally Advanced and Stage IV Breast Cancer
Susie X. Sun
Sarah M. Desnyder
Locally Advanced Breast Cancer
Epidemiology
The definition of locally advanced breast cancer remains heterogeneous and includes tumors that encompass a large portion of the breast, involve the skin and/or chest wall, or have the presence of bulky metastatic disease to regional lymph node. Approximately 12% of breast cancers are found to be locally advanced at the time of diagnosis (1). Survival and recurrence rates vary depending on stage at the time of diagnosis. However, patients with locally advanced breast disease have higher rates of locoregional recurrence, distant metastasis, and lower survival when compared with patients with early breast cancers. The American Cancer Society found that the 5-year survival is 99% for patients with localized disease, 85% for those with regional disease, and 27% for those with distant disease (2).
Diagnosis
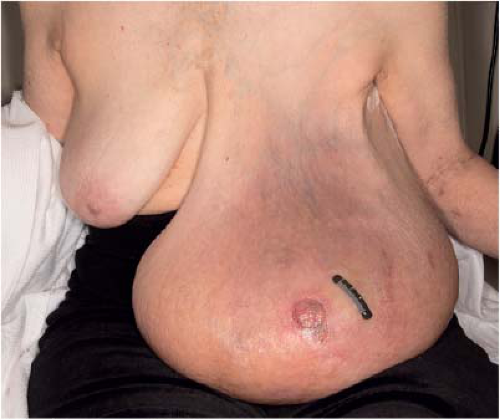
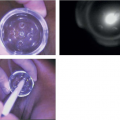
Physical examination is of the utmost importance in the diagnosis of locally advanced breast cancer. A complete breast examination should be performed including inspection and palpation of the breasts and regional nodal basins. The breast examination should be performed with the patient in the upright and supine positions with arm-raising maneuvers. It is important to compare the breasts and note any asymmetry. Special attention is needed to assess for skin changes (dimpling, peau d’orange, redness, and erosions), nipple changes (retraction, flaking, and irritation), and nipple discharge (Fig. 13-1). Palpation of all draining regional lymph node basins should be performed. If a palpable mass is present, a history should be obtained from the patient to determine the length of time the mass has been present and if there have been any changes in the size of the mass, as rapidly growing masses may lead to changes in care.
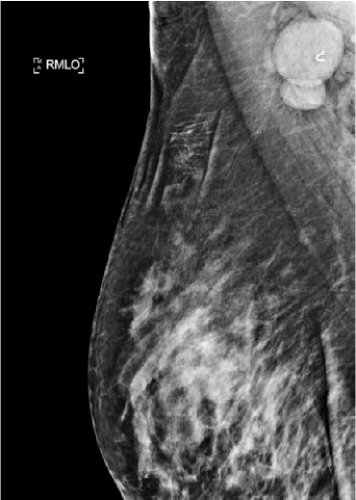
Imaging of the breast and regional lymph nodes should be obtained including mammogram and ultrasound (US) (Fig. 13-2). Core needle biopsy should be performed to acquire tissue from the primary tumor. The biopsy specimen also needs to be evaluated for hormone receptor (HR) status and HER-2 overexpression. For purposes of future treatment planning, a clip needs to be placed within the tumor. If abnormal-appearing lymph nodes are found on imaging, biopsy should be performed via fine-needle aspiration. If a dominant axillary node is biopsied a clip should be placed to identify this node at surgery.
Complete staging workup should be performed to evaluate for distant metastatic disease. This consists of a complete blood count, comprehensive metabolic panel including liver function tests and alkaline phosphatase, CT scan of the chest and abdomen, and bone scan. If there is clinical suspicion for brain metastasis, an MRI of the head may be obtained. Per National Comprehensive Cancer Network (NCCN) guidelines, PET scan remains optional and its use is at the discretion of the provider (3). Patients with breast cancer, especially those with
locally advanced disease, need to be evaluated by a multidisciplinary team including a breast surgeon, a medical oncologist, a radiation oncologist, a radiologist, and a plastic surgeon. A multidisciplinary team approach is critical for treatment planning, especially for patients with locally advanced breast cancer.
locally advanced disease, need to be evaluated by a multidisciplinary team including a breast surgeon, a medical oncologist, a radiation oncologist, a radiologist, and a plastic surgeon. A multidisciplinary team approach is critical for treatment planning, especially for patients with locally advanced breast cancer.
Systemic Treatment
Neoadjuvant systemic therapy has become a key component in the treatment of locally advanced breast cancer. While studies have shown that there is no significant difference in survival between patients who received neoadjuvant and adjuvant treatment, there are several advantages to neoadjuvant systemic therapy (4). For example, a previously inoperable breast cancer may become operable after neoadjuvant treatment. In randomized controlled trials, more patients who received neoadjuvant systemic therapy became candidates for breast-conserving therapy compared to those who received adjuvant treatment (5). Lastly, neoadjuvant systemic therapy provides information about the biology of the cancer by allowing assessment of an in vivo response to treatment. Pathologic response to treatment has been shown to be associated with an improvement in disease-free and overall survival (6). The specific regimen for systemic therapy varies depending on tumor receptor status. In general, regimens containing both an anthracycline and a taxane are recommended for HER-2–negative disease. For HER-2–positive disease, protocols with HER-2 targeted therapy (trastuzumab, pertuzumab) in combination with an anthracycline have been shown to be associated with improved rates of clinical and pathologic response (7,8).
Surgical Treatment
Although surgical management of locally advanced breast cancer has historically consisted of modified radical mastectomy, breast-conserving surgery is being performed more frequently for patients with a favorable response to neoadjuvant systemic therapy. For patients who received neoadjuvant treatment and were candidates for breast-conserving surgery, there was no difference in overall or disease-free survival for those who underwent mastectomy compared to those who received breast-conserving therapy (9). Eligibility for breast-conserving surgery includes tumor size that would not result in unacceptable cosmesis following excision, unifocal disease, minimal skin involvement, patient desire for breast conservation, and no contraindications to radiation therapy. If involvement of the nipple areolar complex is present, a central segmentectomy with removal of the nipple areolar complex can be performed. When performing mastectomy or partial mastectomy, it is important to resect all grossly abnormal–appearing skin. If there is involvement of the underlying pectoralis muscle or the chest wall, resection of the muscle or full-thickness chest wall resection may be indicated. In these cases, multidisciplinary care with a thoracic surgeon and a plastic surgeon is important.
For clinically node-negative patients, sentinel lymph node biopsy (SLNB) has been showed to be safe and effective for axillary staging. Studies, including the NSABP B-32, have shown that SLNB has low false-negative rates with no difference in overall and disease-free survival when compared with axillary lymph node dissection (ALND) (10,11). Of note, clinically node-negative patients who receive neoadjuvant systemic therapy followed by breast-conserving therapy were not evaluated as part of the American College of Surgeons Oncology Group (ACOSOG) Z011 trial and thus this patient cohort should undergo completion ALND if they are found to have positive nodes at the time of SLNB (3,12).
Patients with clinically involved axillary lymph nodes have historically undergone ALND. For patients with locally advanced breast cancer, 20% to 40% will achieve a complete clinical response (7,13,14). For these patients, the possibility for omission of ALND has been evaluated (7,15). If bulky lymph node disease remains after systemic therapy or if the patient initially presents with high-volume tumor burden within the axillary lymph nodes (>4 abnormal-appearing lymph nodes on preoperative imaging) then ALND is indicated. In select patients with low-volume lymph node disease, especially for those
with clinical complete response after neoadjuvant systemic therapy, consideration may be given to omission of ALND. These patients may also qualify for enrollment in the Alliance A011202 trial which is randomizing patients with clinical N1 disease at time of diagnosis and residual nodal disease after neoadjuvant systemic therapy to completion ALND with nodal irradiation or no further axillary surgery with axillary and nodal irradiation. Optimal management of the axilla after neoadjuvant therapy remains highly debated and is discussed in more detail in Chapter 27.
with clinical complete response after neoadjuvant systemic therapy, consideration may be given to omission of ALND. These patients may also qualify for enrollment in the Alliance A011202 trial which is randomizing patients with clinical N1 disease at time of diagnosis and residual nodal disease after neoadjuvant systemic therapy to completion ALND with nodal irradiation or no further axillary surgery with axillary and nodal irradiation. Optimal management of the axilla after neoadjuvant therapy remains highly debated and is discussed in more detail in Chapter 27.
Stage IV Breast Cancer
In the United States, approximately 5% of patients are found to have stage IV breast cancer at the time of initial diagnosis (2). However, the incidence of stage IV disease is higher for African American patients at approximately 10%. This has been attributed to differences in socioeconomic status and overestimation in the rate of screening mammography use in African American patients (16). Five-year survival for patients with distant metastasis is 25%. However, this is also lower at 15% for African American patients (16).
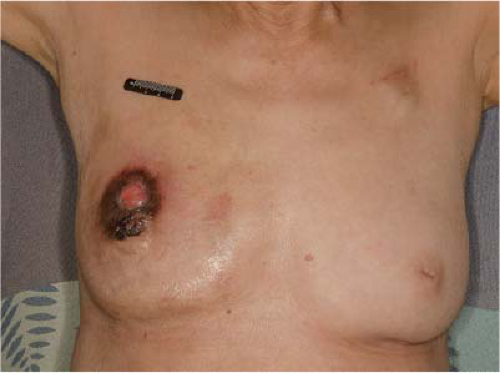
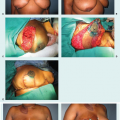
Traditionally, the treatment for stage IV breast cancer has consisted mainly of systemic therapy with surgical resection reserved for palliation. For patients with stage IV disease who have a fungating mass, ulceration of the skin, bleeding, or pain, palliative surgery is clearly indicated (Fig. 13-3). More recently, studies have evaluated the role of surgery for stage IV breast cancer outside of palliation. While surgical resection has not been formally included in the NCCN guidelines for the treatment of stage IV breast cancer, the group recently added a statement acknowledging that surgery for metastatic breast cancer is the subject of ongoing investigation and that the performance of breast surgery is reasonable in select patients with a favorable response to initial systemic therapy (3).
Aggressive local therapy has been shown to improve survival in the setting of metastatic cancer in nonbreast disease sites including renal cell cancer, colorectal cancer, gastric cancer, and ovarian cancer (17,18,19,20). With multimodality systemic treatment, survival for patients with stage IV breast cancer has improved within the past 10 to 20 years, thus eliciting increased interest in evaluating the benefit of surgery for this patient cohort. The underlying principle for the potential benefit of surgical resection at a biologic level has been studied. Hypotheses include decreasing the risk of metastasis by removing the cancer stem cells within the primary tumor, limiting tumor-related immunosuppression by decreasing tumor burden, and disrupting the molecular signaling pathways between the primary tumor and the metastatic sites (21,22,23).
Several retrospective studies have shown a survival advantage associated with resection of the primary tumor. Khan et al. used the National Cancer Database to evaluate 16,203 patients from 1990 to 1993 (24). They found that the 3-year survival for patients who underwent no surgery was 17.3% compared to 27.7% in those who underwent segmental mastectomy, and 31.8% for those who underwent mastectomy. Independent covariates associated with survival included metastatic burden, the presence of visceral metastasis, and the use of systemic therapy. For patients who underwent resection, superior 3- and 5-year survival was seen in women who had negative margins compared to those with involvement of the margins (24). A study from The University of Texas MD Anderson Cancer Center also showed improved survival for patients who underwent resection of their primary tumor (25). Additionally, they found that patients in the surgical group were more likely to have only one site of distant metastasis, be younger, have lower nodal tumor burden, have HER-2–positive disease, and to be treated with systemic therapy. A recent meta-analysis that included 30 retrospective studies showed improved survival following primary tumor resection with a hazard ratio of 0.65, p < 0.001 (26). Sensitivity analysis showed that surgery was beneficial in patients with one site of metastatic disease, bone metastasis only, and negative surgical margins. Within these retrospective studies, patients who underwent surgery have been shown to be younger, have fewer comorbidities, and have less tumor burden compared with patients who did not undergo surgery. Studies have attempted to account for the aforementioned selection bias by performing propensity score–matched analyses, after which the survival advantage associated with surgical resection was no longer present (27,28). Improved survival has also been shown in patients who underwent surgery only if they had a favorable response to systemic therapy (28,29). The role of axillary surgery has also been explored. Two retrospective studies have shown improved survival in patients who underwent ALND at the time of resection of their
primary tumor compared to those who underwent SLNB alone or no axillary surgery (29,30).
primary tumor compared to those who underwent SLNB alone or no axillary surgery (29,30).
Because patient selection may be a strong bias leading to the observed survival benefit associated with resection of the primary tumor for patients with stage IV breast cancer, randomized controlled trials are currently in progress. The Translational Breast Cancer Research Consortium (TBCRC) 013 is a multicenter prospective registry study evaluating the role of surgical excision of the primary tumor along with response to systemic therapy, incidence of disease progression, and molecular characteristics of the tumor (31). A total of 127 patients from 14 sites were categorized into two cohorts, stage IV with an intact primary tumor (cohort A, n = 112) or metastasis within 3 months of primary surgery (cohort B, n = 15). All patients received first-line neoadjuvant systemic therapy. Initial results presented at the San Antonio Breast Cancer Symposium in 2013 showed superior survival in cohort B compared with cohort A (100% vs. 84%, p = 0.03). When patients in both cohort A and cohort B were considered together, resection of the primary tumor was associated with improved 2-year overall survival (96% vs. 74%, p = 0.002). No survival difference was seen in cohort A patients with response (partial, complete response, or stable disease) to systemic therapy who then underwent elective surgery when compared to those who did not (94% vs. 92%, p = 0.5). Updated results for this trial were presented at the 2016 American Society of Clinical Oncology (ASCO) annual meeting (32). Patients with intact primaries were classified by response to systemic therapy and the 3-year overall survival was better for responders compared to nonresponders (78% vs. 24%, p < 0.001). Again, among patients who responded to systemic therapy, no difference in 3-year survival was seen between those who underwent resection of their primary tumor and those who did not (77% vs. 76%, p = 0.85). Patients who had surgery were more likely to have larger tumors, single-organ metastasis, and receive first-line chemotherapy.
Two prospective randomized controlled trials have been published evaluating the role of surgical resection of the primary tumor in the setting of stage IV breast cancer. The MF07-01 is a multicenter trial conducted in Turkey comparing initial locoregional surgery followed by systemic treatment with systemic therapy alone (33). While there was no survival difference seen between the two groups at 3 years (60% vs. 51%, p = 0.10), overall survival was higher for the locoregional surgery group at 5 years (41.6% vs. 24.4%, p = 0.005). This study enrolled 274 patients with 138 in the surgery arm and 136 in the systemic treatment–only arm. Most of the patients who had initial locoregional surgery underwent mastectomy with ALND followed by systemic therapy. The systemic therapy regimen and rates of use were similar between the two groups. Subgroup analysis of patients who underwent locoregional surgery showed that improved survival was associated with HR positivity, HER-2–negative disease, age less than 55, and solitary bone metastasis. Additional analysis of the solitary bone metastasis group showed improved overall 5-year survival in the locoregional surgery group (51.7% vs. 29.3%, p = 0.04). Also of note, locoregional disease progression or relapse, defined as clinical or radiologic progression of the primary tumor, ulceration, bleeding, or new locoregional lesions, was 1% in the locoregional surgery group compared to 11% in the systemic therapy–alone group (p = 0.001). When patients with multiple pulmonary or liver metastases were evaluated, 3-year survival was significantly lower in the surgery group (31% vs. 67%, p = 0.05). While this study shows improved survival associated with surgical resection of the primary tumor, there are several limitations that warrant consideration. First, it is important to highlight that the patient cohort in the MF07-01 differs from that of the TBCRC 013 as it reflects patients who underwent upfront surgery and thus included those who would have been considered nonresponders in the TBCRC 013. With recent improvements in systemic therapy, especially with targeted therapies, it is difficult to justify not giving patients neoadjuvant systemic treatment in the setting of metastatic breast cancer, thus limiting the applicability of these study results. Additionally, patient and tumor characteristics such as receptor status were not taken into account during randomization leading to a higher proportion of patients with HR-positive disease in the surgery arm (85.5% vs. 71.8%, p = 0.01) and a higher rate of triple-negative disease in the systemic treatment–only arm (17.4% vs. 7.3%, p = 0.01). This indicates that patients in the surgery arm likely had more indolent disease which contributed to the observed survival advantage. In summary, the MF07-01 study suggests that patients who may derive benefit from resection of the primary tumor are younger with HR-positive, HER-2–negative disease. Those with triple-negative disease and/or large metastatic burden, such as liver or pulmonary metastases, are less likely to benefit from removal of the primary tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree