Initially popularized for the treatment of strabismus and blepharospasm, injection of botulinum neurotoxin has become the most commonly performed cosmetic treatment in the United States. Injection techniques have been particularly well-studied in the midface and periocular region, and patient satisfaction tends to be very high. We review the salient differences among available neurotoxins, how to optimally reconstitute them, how to inject the forehead, glabella, lateral canthal lines (“crow’s feet”), infralid region, and transverse nasal lines (“bunny lines”), how to sculpt the brow, and how to manage potential complications.
Key points
- •
Three neurotoxin formulations are approved for cosmetic use in the United States: onabotulinumtoxinA (Botox), abobotulinumtoxinA (Dysport), and incobotulinumtoxinA (Xeomin).
- •
Selection is dictated by injector and patient preference, patient history, and the presence of allergies, among other considerations.
- •
No 2 toxins are exactly the same unit for unit, and there is no universally accepted formula for unit conversion.
- •
Complications of botulinum neurotoxin injection, such as eyelid ptosis, brow ptosis, and double vision, can be reduced with appropriate reconstitution and injection techniques.
- •
The trend has been toward an individualized, sculpted approach that preserves baseline facial animation and accounts for the gender, ethnicity, individual preference, and professional needs.
Introduction
The concept of therapeutic botulinum toxin (BoNT) injection was ushered in by Dr Allen Scott’s landmark study in the late 1970s, which demonstrated safety and efficacy for the treatment of adult strabismus. The US Food and Drug Administration (FDA) approved the first commercial BoNT formulation, onabotulinumtoxinA, for the treatment of strabismus and blepharospasm in 1989. After Carruthers and Carruthers first recognized the dramatic reduction of glabellar rhytids in patients treated for essential for blepharospasm, however, it took more than a decade for the FDA to extend approval to esthetic indications, long after the toxin was already popularized for cosmetic use.
Since the approval of cosmetic Botox in 2002, the number of annual treatments has skyrocketed, making BoNT injections the top nonsurgical esthetic enhancement worldwide for more than a decade. Facial injections were the single most common cosmetic procedure in 2011, accounting for 41% of all esthetic interventions performed in the United States. According to 2013 data from the American Society of Plastic Surgeons, 6.3 million cosmetic BoNT A injections were performed, nearly 3 times the number of dermal filler treatments. Market expansion continues at an impressive rate, particularly among men. The number of males seeking cosmetic injections has grown by 268% since 2000. Satisfaction and rates of return for repeat injections are very high, making it both an excellent source of practice revenue and an effective way of introducing patients to other cosmetic treatments.
Seven distinct serotypes of BoNT (A–G) have been isolated from different strains of Clostridium botulinum . Only BoNT-A and BoNT-B, which function exclusively in cholinergic neurons, have been approved for clinical use. There are currently 4 neurotoxin formulations available for injection in the United States: onabotulinumtoxinA (onaBoNT-A, Botox, Allergan, Inc, Irvine, CA), abobotulinumtoxinA (aboBoNT-A, Dysport, Galderma Laboratories, L.P., Fort Worth, TX), incobotulinumtoxinA (incoBoNT-A, Xeomin, Merz Pharmaceuticals, L.L.C., Greensboro, NC), and rimabotulinumtoxinB (rimaBoNT-B, Myobloc, Solstice Neurosciences, Inc, San Francisco, CA). All 3 BoNT-A preparations are approved for the correction of moderate-to-severe glabellar lines but only onaBoNT-A is approved for lateral canthal lines; all other uses remain off label.
In nature, BoNT consists of a 150-kDa core. With the exception of incoBoNT-A, this core is surrounded by varying amounts of nontoxic complexing proteins. These proteins are thought to stabilize the neurotoxin, protecting it from pH and temperature fluctuations as well as lytic enzymes. Although these accessory proteins confer an obvious advantage to the Clostridial bacillus during gastrointestinal transit, the benefits are less clear for therapeutic use. In fact, there is mounting evidence that the presence of complexing proteins merely increases neurotoxin antigenicity without prolonging product shelf life. Accordingly, most manufacturers have worked to reduce the foreign protein load present in each unit of BoNT.
BoNT functions at the level of the neuromuscular junction by blocking acetylcholine release, thus decreasing contraction of the motor unit. It is taken up in the presynaptic terminal via receptor-mediated endocytosis. In the acidic environment of the endosome, a disulfide bond is cleaved, separating the core protein into heavy and light chains, which are the active moieties. By irreversibly inhibiting components of the SNARE complex, BoNT prevents nontoxic exocytosis; BoNT-A cleaves SNAP-25, whereas BoNT-B cleaves synaptobrevin.
With ongoing turnover at the neuromuscular junction, however, contractile function begins to return after several weeks, and usually attains pretreatment strength by 6 months. From a cosmetic and therapeutic standpoint, treatments generally remain effective for 3 to 4 months, and there is no evidence of tachyphylaxis in the majority of patients.
Introduction
The concept of therapeutic botulinum toxin (BoNT) injection was ushered in by Dr Allen Scott’s landmark study in the late 1970s, which demonstrated safety and efficacy for the treatment of adult strabismus. The US Food and Drug Administration (FDA) approved the first commercial BoNT formulation, onabotulinumtoxinA, for the treatment of strabismus and blepharospasm in 1989. After Carruthers and Carruthers first recognized the dramatic reduction of glabellar rhytids in patients treated for essential for blepharospasm, however, it took more than a decade for the FDA to extend approval to esthetic indications, long after the toxin was already popularized for cosmetic use.
Since the approval of cosmetic Botox in 2002, the number of annual treatments has skyrocketed, making BoNT injections the top nonsurgical esthetic enhancement worldwide for more than a decade. Facial injections were the single most common cosmetic procedure in 2011, accounting for 41% of all esthetic interventions performed in the United States. According to 2013 data from the American Society of Plastic Surgeons, 6.3 million cosmetic BoNT A injections were performed, nearly 3 times the number of dermal filler treatments. Market expansion continues at an impressive rate, particularly among men. The number of males seeking cosmetic injections has grown by 268% since 2000. Satisfaction and rates of return for repeat injections are very high, making it both an excellent source of practice revenue and an effective way of introducing patients to other cosmetic treatments.
Seven distinct serotypes of BoNT (A–G) have been isolated from different strains of Clostridium botulinum . Only BoNT-A and BoNT-B, which function exclusively in cholinergic neurons, have been approved for clinical use. There are currently 4 neurotoxin formulations available for injection in the United States: onabotulinumtoxinA (onaBoNT-A, Botox, Allergan, Inc, Irvine, CA), abobotulinumtoxinA (aboBoNT-A, Dysport, Galderma Laboratories, L.P., Fort Worth, TX), incobotulinumtoxinA (incoBoNT-A, Xeomin, Merz Pharmaceuticals, L.L.C., Greensboro, NC), and rimabotulinumtoxinB (rimaBoNT-B, Myobloc, Solstice Neurosciences, Inc, San Francisco, CA). All 3 BoNT-A preparations are approved for the correction of moderate-to-severe glabellar lines but only onaBoNT-A is approved for lateral canthal lines; all other uses remain off label.
In nature, BoNT consists of a 150-kDa core. With the exception of incoBoNT-A, this core is surrounded by varying amounts of nontoxic complexing proteins. These proteins are thought to stabilize the neurotoxin, protecting it from pH and temperature fluctuations as well as lytic enzymes. Although these accessory proteins confer an obvious advantage to the Clostridial bacillus during gastrointestinal transit, the benefits are less clear for therapeutic use. In fact, there is mounting evidence that the presence of complexing proteins merely increases neurotoxin antigenicity without prolonging product shelf life. Accordingly, most manufacturers have worked to reduce the foreign protein load present in each unit of BoNT.
BoNT functions at the level of the neuromuscular junction by blocking acetylcholine release, thus decreasing contraction of the motor unit. It is taken up in the presynaptic terminal via receptor-mediated endocytosis. In the acidic environment of the endosome, a disulfide bond is cleaved, separating the core protein into heavy and light chains, which are the active moieties. By irreversibly inhibiting components of the SNARE complex, BoNT prevents nontoxic exocytosis; BoNT-A cleaves SNAP-25, whereas BoNT-B cleaves synaptobrevin.
With ongoing turnover at the neuromuscular junction, however, contractile function begins to return after several weeks, and usually attains pretreatment strength by 6 months. From a cosmetic and therapeutic standpoint, treatments generally remain effective for 3 to 4 months, and there is no evidence of tachyphylaxis in the majority of patients.
Treatment goals
The superficial facial mimetic muscles insert directly onto the undersurface of the skin; repetitive contraction therefore causes characteristic furrows (rhytids) to form perpendicular to the direction of contraction. Although injection of BoNT does not eliminate static wrinkling, it can reduce dramatically the appearance of hyperdynamic lines in the midface and periocular region via selective chemodenervation.
Since the introduction of cosmetic Botox, treatment goals and therapeutic endpoints have continued to evolve. The trend has been away from a one-size-fits-all ‘frozen’ appearance, and toward a more subtle, individualized approach. It is important to account for the gender, ethnicity, individual preference, and professional needs of each patient. With careful appraisal of each patient’s anatomy, injection can produce a harmonious appearance in which facial animation is preserved. By selectively targeting depressors in preference to elevators, modest lifting and sculpting can also be achieved.
Synergies can be obtained by combining BoNT injection with dermal fillers, cutaneous lasers, retinoids, and facial plastic surgery. Because most rhytids have both static and dynamic contributions, the optimal approach often combines neurotoxin and dermal filler injections. A number of studies report improved patient satisfaction and more durable results when BoNT and hyaluronic acid treatments are administered in tandem.
Preoperative planning and preparation
Selecting a Product
Three different formulations of BoNT-A are available for cosmetic injection in the United States. RimaBoNT-B has a demonstrably shorter half-life and is only FDA approved for treatment of cervical dystonia; its use is therefore typically limited to secondary nonresponders. Comparing the efficacy of onaBoNT-A, aboBoNT-A, and incoBoNT-A is difficult because available head-to-head trials have enrolled relatively small numbers of subjects and are often industry sponsored. On the whole, these products have more similarities than differences; variations in potency, speed of onset, therapeutic duration, and immunogenicity are relatively minor when compared with BoNT-B. Important factors in product selection, therefore, include physician comfort and familiarity with the product being injected, as well as patient preference and history.
The units used to measure different formulations of BoNT are not directly interchangeable. This designation is shorthand for “mouse unit,” or the median intraperitoneal dose of toxin that results in death of a laboratory mouse (LD50). Because each manufacturer uses a slightly different protocol, a corrective factor often has to be employed when a physician familiar with injecting one product switches to another. Fortunately, relatively reliable consensus nomograms have now been established for all available products. Units of onaBoNT-A and incoBoNT-A are generally interchanged in a 1:1 ratio, whereas 2.5 to 3 times the number of units are generally required for aboBoNT-A, and 40 to 50 times the number of units for rimaBoNT-B.
Cosmetic onabotulinumtoxinA (Botox, Allergan, Inc) was approved for the treatment of moderate-to-severe glabellar rhytids in 2002 and then became the first neurotoxin FDA approved for the correction of lateral canthal lines in 2013. It is available in 50- and 100-unit vials, and has a stable shelf life of 3 years at 2°C to 8°C. According to the manufacturer, it should be stored at 2°C to 8°C after reconstitution and used within 24 hours. The package insert recommends dilution of 100 units in 2.5 mL of normal saline (40 U/mL). Some injectors prefer to use higher concentrations (≤100 U/mL) in areas where there is a more significant risk of side effects from diffusion, and lesser concentrations (20–25 U/mL) for areas, such as the forehead, where more diffusion may produce a ‘softer’ look. In 1997, the Botox formula was altered to reduce the concentration of complexing proteins; product immunogenicity has decreased measurably since this change was made.
IncobotulinumtoxinA (Xeomin, Merz Pharmaceuticals, L.L.C.) was approved by the FDA for the treatment of moderate-to-severe glabellar rhytids in 2011. It is also available in 50- and 100-unit vials. According to the manufacturer, unreconstituted product is stable for 3 years at temperatures ranging from –20°C to 25°C. Independent studies suggest that the shelf life may be up to 4 years at room temperature. It is shipped without dry ice, and can be transported between offices without refrigeration. Once reconstituted, it is certified as stable for 24 hours at 2°C to 8°C. Most practitioners use the same dilutions and dosing as for onaBoNT-A. A variety of industry-sponsored noninferiority trials also suggest comparable onset of action and duration compared with onaBoNT-A. The principal advantage of incoBoNT-A seems to be the absence of complexing proteins, which theoretically reduces the risk of immunogenicity and secondary nonresponse.
AbobotulinumtoxinA (Dysport, Galderma Laboratories, L.P.) was FDA approved for the treatment of moderate-to-severe glabellar rhytids in 2009 and comes in 300-unit vials. The reported shelf life is 2 years. It is shipped on dry ice and should be stored at 2°C to 8°C until used. The manufacturer recommends that it be injected within 4 hours of reconstitution. The 300-unit vial is typically diluted in between 1.5 and 3 mL of sodium chloride (yielding a concentration of 100–200 U/mL). The majority of practitioners inject 2.5 to 3 units of aboBoNT-A for every 1 unit of onaBoNT-A or incoBoNT-A. The main consensus observation regarding aboBoNT-A is that onset tends to be slightly more rapid, but duration of action is slightly shorter when compared with other BoNT-A formulations. Some data suggest that aboBoNT-A may diffuse slightly more than other comparably diluted products, although this has not been linked to a higher incidence of adverse effects. It is also important to know that aboBoNT-A may contain trace amounts of cow’s milk protein because it is manufactured with lactose, and patients with true allergy (vs intolerance) should not be injected.
RimabotulinumtoxinB (Myobloc, Solstice Neurosciences, Inc) was FDA approved for cervical dystonia, and has not subsequently been extended to any cosmetic indications. In contrast with all BoNT-A formulations, it comes as a ready-to-use sterile liquid with an unrefrigerated shelf life of 9 months and a refrigerated life of up to 4 years. Owing to its acidic nature (pH 5.6), injections tend to be more uncomfortable. It also has a faster onset and considerably shorter average duration of efficacy (generally 6–10 weeks). Because of this, cosmetic use is typically limited to rare secondary nonresponders who have developed neutralizing antibodies to BoNT-A. Similar reductions in the concentration of complexing proteins have been achieved owing to refinements in the manufacturing process, and the rates of immune-related failure for cervical dystonia (which requires very high doses of neurotoxin) have fallen from 5% to 17% to 1.2%.
Reconstitution, Handling, and Storage
All BoNT-A formulations are delivered as lyophilized powder in a vacuum-sealed vial. We draw up the chosen diluent in a sterile 3-mL syringe with a blunt cannula or large filter tip needle, which is then used to pierce the rubber stopper of the vial after it is cleaned with an alcohol pad. The vacuum should draw in the majority of diluent automatically, and mild pressure can be used to inject the remaining saline as needed ( Fig. 1 ). We then roll the vial gently to dissolve the product, taking care not to agitate it or cause foam to develop. Despite recent studies demonstrating that rough handling and agitation may not affect toxin potency to the degree once feared, we still believe it prudent to exercise caution. In the case of incoBoNT-A, Merz Pharmaceuticals recommends that the vial be inverted to ensure that any leftover product under the cap is dissolved, and we commonly perform this maneuver for all toxins during reconstitution. Exposure of product to direct sunlight should also be avoided.
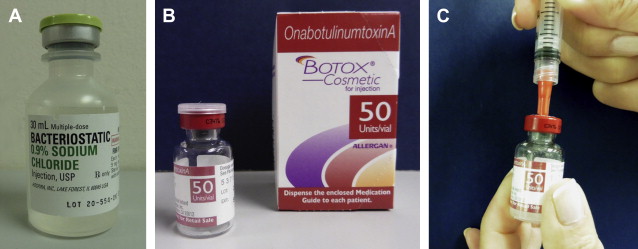
We then remove the metal cap and stopper from the vial using a household can opener or large Webster needle holder. This obviates the need to draw up and inject with separate needles. Even with smaller 50-mL vials, it is possible to obtain product directly with 31-gauge diabetic syringes, although care should be taken not to blunt the tip on the bottom of the glass container. Use of a hubless syringe with an attached needle minimizes waste. The vial can later be sealed with the rubber stopper if there is residual product to refrigerate.
All manufacturers recommend reconstitution with preservative-free sterile saline, but a 2004 consensus panel stated that preserved saline is the preferred diluent for the reconstitution of neurotoxins. It contains 0.9% benzyl alcohol, which has a mild analgesic effect and does not seem to compromise the potency or longevity of the toxin (see Fig. 1 A). A number of authors have demonstrated that BoNT-A can be reconstituted with 1% or 2% lidocaine with epinephrine, but we have not found this to be necessary.
All neurotoxin vials are labeled and marketed for single use, and injection is recommended within 4 to 24 hours. Despite this, the majority of clinicians store product that has been reconstituted for significantly longer periods of time. The current consensus is that product refrigerated at 2°C to 8°C for up to 6 weeks remains safe and effective. There is some evidence that product may be frozen for up to 6 months without an appreciable impact on potency. All cooled solutions, however, should be allowed time to equilibrate to room temperature to prevent injection site discomfort. Regardless of the product, it is recommended that, after reconstitution, the solution be refrigerated rather than left at room temperature.
Product Dilution
Different physicians prefer a variety of toxin concentrations, and may even modify the dilution based on the location of injection. In general, more concentrated product has less risk of diffusion and may last longer, but some injectors prefer the ‘softer’ look that is achieved with higher diluent volumes in the forehead and lateral canthal region. There are conflicting data regarding the extent to which greater diffusion with higher diluent volumes may predispose to additional complications. Carruthers and colleagues injected 10, 20, 33.3, or 100 U/mL of onaBoNT-A in the glabella (20 patients per group) and found no difference in therapeutic success or complications, although all 6 cases of eyebrow ptosis developed in the groups receiving more dilute injections.
We have found the use of a 50 U/mL concentration for both onaBoNT-A and incoBoNT-A to be a simple and effective compromise for all of our periocular injections. Using this dilution, a 0.3-mL Ultrafine II Diabetic syringe (Becton Dickenson, Franklin Lakes, NJ) holds 15 units; 0.1 mL contains 5 units, and 0.05 mL contains 2.5 units. Using multiples of 5 and 2.5 makes calculating and injecting easy, and is convenient not to have to draw up 2 different concentrations for the forehead and glabella.
Patient preparation and positioning
Contraindications to cosmetic neurotoxin injection are rare, but must be considered in all patients. BoNT is a category C medication in pregnancy and lactation, and it is prudent to avoid elective treatment. Neuromuscular disorders such as myasthenia gravis, Eaton-Lambert syndrome and amyotrophic lateral sclerosis can theoretically be worsened by neurotoxin injections. Neurotoxins should be administered with caution in patients taking medications, such as aminoglycosides, tetracyclines, polymixins, penicillamine, anticholinesterases, and calcium channel blockers, that can theoretically alter conduction at the neuromuscular junction. Hypersensitivity to any known constituent of the formulation (eg, milk protein with Dysport) is also a contraindication.
Zoster reactivation has been described after neurotoxin injections as a rare side effect. We typically reserve prophylactic acyclovir or valacyclovir, however, for patients with known cold sores or other herpetic manifestations, who are undergoing simultaneous treatment with other injectables or lasers.
We use alcohol pads to remove facial oils and makeup from the injection sites. In our practices, chlorhexidine scrubs are used in patients who are undergoing simultaneous dermal filler injections, owing to the higher risk of infection with atypical mycobacteria and the chance of developing biofilms. We rarely find it necessary to apply topical anesthetics such as EMLA (Astra Pharmaceuticals, Westborough, MA) before injection or to add analgesics apart from preserved sterile saline and occasional ice packs.
The patient should be seated upright with his or her head firmly supported by a headrest and at the injector’s shoulder height. The nondominant hand should be used actively to pinch up and protect various parts of the periocular and midfacial anatomy. Particularly in the glabellar region, pinching the corrugators protects the orbital rim and decreases the chance of toxin diffusing into the levator aponeurosis, which can result in blepharoptosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



