Introduction72
INTRODUCTION
MAJOR PSORIASIFORM DERMATOSES
PSORIASIS
Clinical variants
Genetics of psoriasis
Trigger factors
ACE inhibitors
Infliximab
Adalimumab
Interferon-α
Ampicillin
Interleukin-2
Anakinra
Iodine
Antimalarials
Isotretinoin
Beta-blockers
Lithium
Calcium channel blockers
NSAIDs
Carbamazepine
Olanzapine
Celecoxib
Propafenone
Cimetidine
Quinidine
Clonidine
Rofecoxib
Ecstasy
Terbinafine
Fluorescein sodium
Terfenadine
Glibenclamide
Tetracyclines
Icodextrin
Thalidomide
Imiquimod
Urapidil
Indomethacin
Voriconazole
Pathogenesis of psoriasis
*After Bonifati and Ameglio (Int J Dermatol 1999; 38: 241–251).
Pathogenetic cascade
Cytokines involved
Endothelial activation and vascular changes
→
IL-1, IL-6, IL-8, TNF-α, TGF-α/β, IFN-γ, endothelin-1
↓
Lymphocyte recruitment
→
IL-1, IL-8, MCP-1, TNF-α, psoriasin, CD11a/CD18 (LFA-1), ICAM-1
↓
Keratinocyte–lymphocyte interactions
→
IL-1, IL-7, IL-8, TNF-α, IFN-γ, CD11a/CD18
↓
Amplification of inflammatory mechanisms
→
IL-1, IL-2, IL-6, IL-8, IL-12, IL-17, IL-18, IL-23, TNF-α, IFN-γ, amphiregulin, MCP-1 (IL-10 is anti-inflammatory)
↓
Keratinocyte proliferation
→
IL-1, IL-3, IL-6, IL-8, GM-CSF, IFN-γ, TGF-α, EGF, TNF-α, amphiregulin, endothelin-1, insulin growth factor, TGF-β receptors, GRO-α, phospholipase C/protein kinase C system, S100A8, S100A9, G-MCSF
Treatment of psoriasis
Histopathology2.109.539.540.541. and 542.
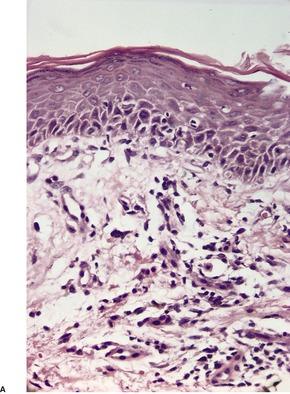
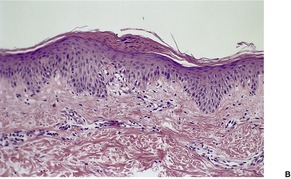
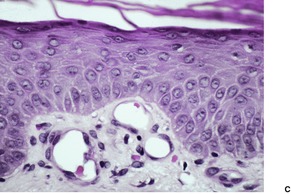
Fig. 4.1
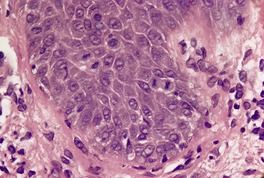
Fig. 4.2
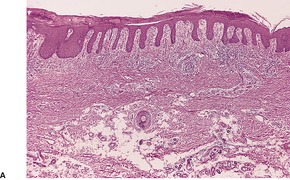
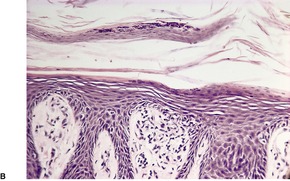
Fig. 4.3
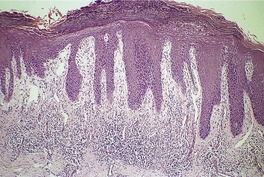
Fig. 4.4
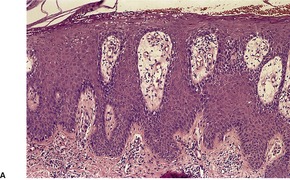
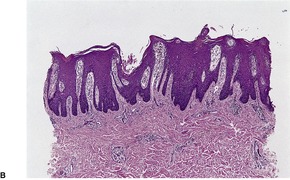
Fig. 4.5
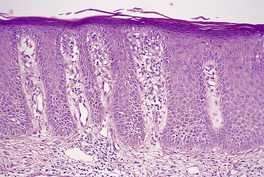
Fig. 4.6
Differential diagnosis
Electron microscopy574
PSORIASIFORM KERATOSIS
Histopathology
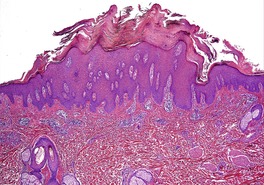
Fig. 4.7
AIDS-ASSOCIATED PSORIASIFORM DERMATITIS
Histopathology
PUSTULAR PSORIASIS
Aceclofenac
Lithium
Alcohol
Malignancy
Beta-blockers
Morphine
Bupropion
NSAIDs
Burns
Nystatin
Calcipotriol
Penicillin and derivatives
Clopidogrel
Phenylbutazone
Corticosteroid withdrawal
Prednisolone
Cyclosporine and its withdrawal
Pregnancy
Doxorubicin
Procaine
Emotional stress
Progesterone
Endocrine/metabolic factors
Sulfonamides
Hydroxychloroquine
Sunlight
Infections
Tanning salons
Infliximab
Terbinafine
Iodides
TNF inhibitors
Treatment of pustular psoriasis
Histopathology674. and 675.
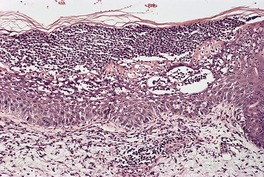
Fig. 4.8
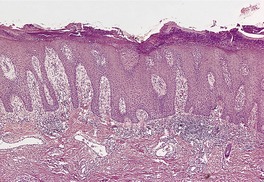
Fig. 4.9
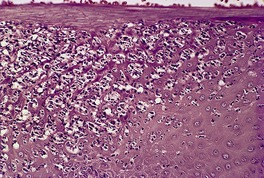
Fig. 4.10
Electron microscopy662
REITER’S SYNDROME
Histopathology
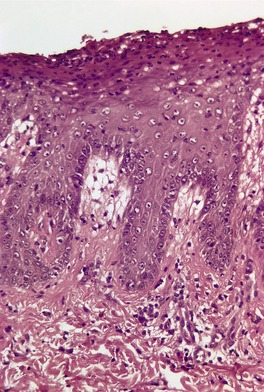
Fig. 4.11
PITYRIASIS RUBRA PILARIS
Treatment of pityriasis rubra pilaris
Histopathology2.716. and 758.
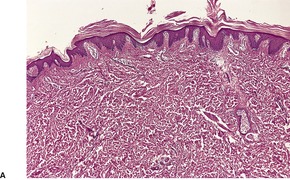
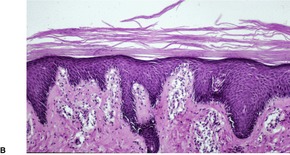
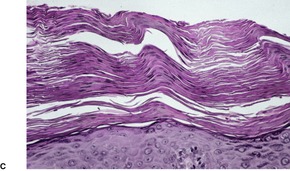
Fig. 4.12
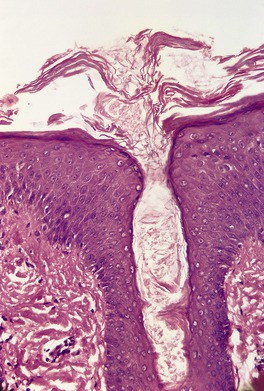
Fig. 4.13
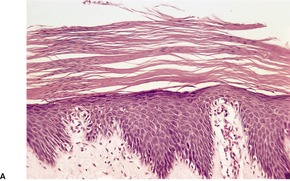
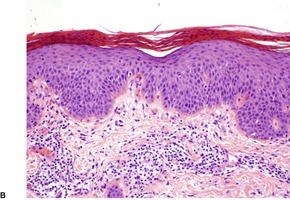
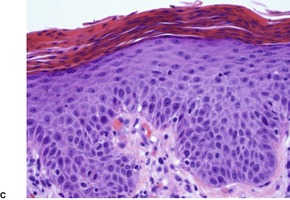
Fig. 4.14
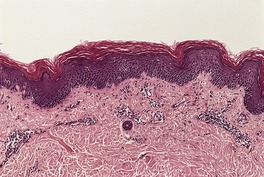
Fig. 4.15
PARAPSORIASIS
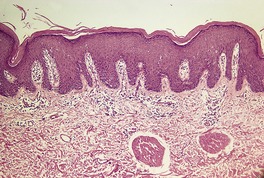
Fig. 4.16
LICHEN SIMPLEX CHRONICUS
Histopathology778. and 785.
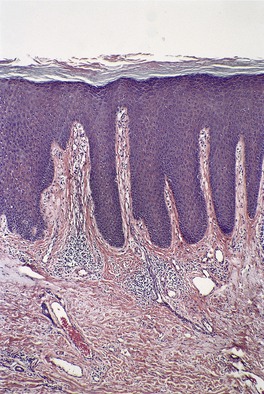
Fig. 4.17
OTHER PSORIASIFORM DERMATOSES
Disease
Histopathological features
Psoriasis
Progressive psoriasiform epidermal hyperplasia, initially mild; mitoses in basal keratinocytes; dilated vessels in dermal papillae; parakeratosis, initially focal and containing neutrophils, later confluent with few neutrophils; thinning of the suprapapillary epidermis
Psoriasiform keratosis
Psoriasis-like, but often more parakeratosis (diffuse or focal)
Pustular psoriasis
Spongiform pustulation overshadows epidermal hyperplasia, except in lesions of some duration when both are present
Reiter’s syndrome
Closely resembles pustular psoriasis; the overlying, thick scale crust often detaches during processing
Pityriasis rubra pilaris
Alternating orthokeratosis and parakeratosis, vertically and horizontally; follicular plugging with parafollicular (lipping) parakeratosis; mild to moderate epidermal hyperplasia; no neutrophil exocytosis
Parapsoriasis
Variable epidermal hyperplasia; the superficial perivascular or band-like infiltrate involves the papillary dermis (‘spills upwards’); some exocytosis/epidermotropism; probably represents early cutaneous lymphoma
Lichen simplex chronicus
Conspicuous psoriasiform hyperplasia, sometimes irregular; prominent granular layer with patchy parakeratosis; thick suprapapillary epidermal plates; thick collagen in vertical streaks in papillary dermis; variable inflammatory infiltrate and plump fibroblasts
Chronic spongiotic dermatitides
Progressive psoriasiform hyperplasia, usually with diminishing spongiosis eventually merging with picture of lichen simplex chronicus; chronic nummular lesions ‘untidy’ with mild exocytosis; eosinophils may be present in nummular and allergic contact lesions; chronic seborrheic dermatitis may mimic psoriasis but no neutrophils, less hyperplasia and sometimes perifollicular parakeratosis
Erythroderma
Variable psoriasiform hyperplasia; usually focal spongiosis; no distinguishing features
Mycosis fungoides
Epidermotropism; papillary dermal infiltrate of lymphocytes with variable cytological atypia
Chronic candidosis and dermatophytoses
Psoriasiform hyperplasia not as regular or as marked as in psoriasis; spongiform pustules or neutrophils in parakeratotic scale; fungal elements may be sparse in candidosis
ILVEN
Papillated psoriasiform hyperplasia with foci of parakeratosis overlying hypogranulosis; often focal mild spongiosis; may have alternating orthokeratosis and parakeratosis in a horizontal direction
Norwegian scabies
Marked orthokeratosis and scale crust; numerous mites, larvae and ova in the keratinous layer
Bowen’s disease (psoriasiform type)
Full thickness atypia of keratinocytes but basal layer sometimes spared; cells sometimes pale staining
Clear cell acanthoma
Pallor of keratinocytes but no atypia; abundant glycogen; some exocytosis of inflammatory cells
Lamellar ichthyosis
Mild psoriasiform hyperplasia with a thick compact or laminated orthokeratin layer overlying a prominent granular layer
Pityriasis rosea (herald patch)
Mild psoriasiform hyperplasia; spongiosis and exocytosis of lymphocytes leading to ‘mini-Pautrier simulants’; focal parakeratosis
Pellagra, acrodermatitis enteropathica and glucagonoma syndrome
Mild to moderate psoriasiform hyperplasia; the upper epidermis shows pallor and ballooning progressing sometimes to necrosis, vesiculation or pustulation (not in pellagra); confluent parakeratosis overlying these changes; many cases of pellagra show mild, even non-specific changes
Secondary syphilis
Superficial and deep dermal infiltrate which often includes plasma cells; may have lichenoid changes or granuloma formation in late stages
SUBACUTE AND CHRONIC SPONGIOTIC DERMATITIDES
Histopathology
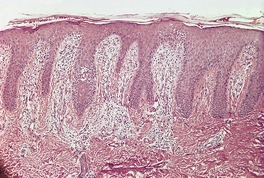
Fig. 4.18
ERYTHRODERMA
Histopathology
MYCOSIS FUNGOIDES
Histopathology
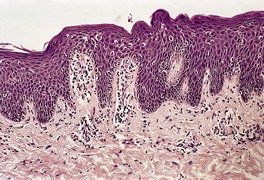
Fig. 4.19
CHRONIC CANDIDOSIS AND DERMATOPHYTOSES
Histopathology
INFLAMMATORY LINEAR VERRUCOUS EPIDERMAL NEVUS
Histopathology
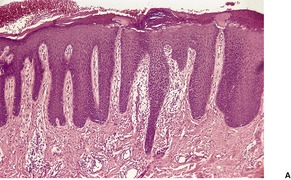
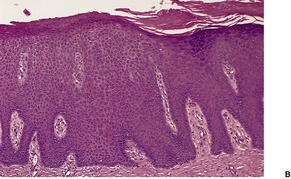
Fig. 4.20
NORWEGIAN SCABIES
Histopathology
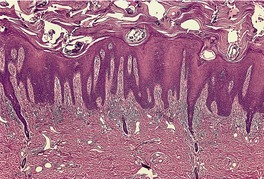
Fig. 4.21
BOWEN’S DISEASE
Histopathology
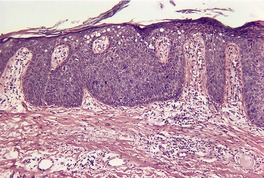
Fig. 4.22
CLEAR CELL ACANTHOMA
Histopathology
LAMELLAR ICHTHYOSIS
Histopathology
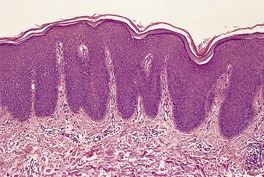
Fig. 4.23
PITYRIASIS ROSEA
Histopathology
PELLAGRA
Histopathology
ACRODERMATITIS ENTEROPATHICA
Histopathology
GLUCAGONOMA SYNDROME
Histopathology
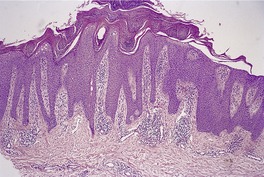
Fig. 4.24
SECONDARY SYPHILIS
Histopathology
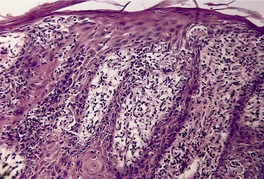
Fig. 4.25
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The psoriasiform reaction pattern
Other psoriasiform dermatoses87
Subacute and chronic spongiotic dermatitides87
Erythroderma88
Mycosis fungoides89
Chronic candidosis and dermatophytoses89
Inflammatory linear verrucous epidermal nevus89
Norwegian scabies89
Bowen’s disease (psoriasiform variant)90
Clear cell acanthoma90
Lamellar ichthyosis90
Pityriasis rosea (‘herald patch’)90
Pellagra90
Acrodermatitis enteropathica91
Glucagonoma syndrome91
Secondary syphilis91
The psoriasiform reaction pattern is defined morphologically as the presence of epidermal hyperplasia with elongation of the rete ridges in a regular manner. This definition encompasses a heterogeneous group of dermatological conditions. This morphological concept is much broader than the pathogenetic one, outlined by Pinkus and Mehregan. 1 They considered the principal features of the psoriasiform tissue reaction to be the formation of a suprapapillary exudate with parakeratosis, secondary to the intermittent release of serum and leukocytes from dilated blood vessels in the papillary dermis (the so-called ‘squirting papilla’).
The increased mitotic activity of the epidermis which results in the elongated rete ridges and the psoriasiform epidermal hyperplasia is presumed to be secondary to the release of various mediators from the dilated vessels in the papillary dermis in psoriasis. These aspects are discussed in further detail below. The epidermal hyperplasia in lichen simplex chronicus may be related to chronic rubbing and irritation, while in Bowen’s disease there is increased mitotic activity of the component cells. In many of the conditions listed the exact pathogenesis of the psoriasiform hyperplasia remains to be elucidated.
Psoriasis is the prototype of the psoriasiform reaction pattern, but it should be noted that early lesions of psoriasis and pustular psoriasis show no epidermal hyperplasia, although there is evidence of a ‘squirting papilla’ in the form of dilated vessels and exocytosis of inflammatory cells with neutrophils collecting in the overlying parakeratotic scale.
The major psoriasiform dermatoses – psoriasis, psoriasiform keratosis, pustular psoriasis, Reiter’s syndrome, pityriasis rubra pilaris, parapsoriasis and its variants, and lichen simplex chronicus – will be considered first. 2 The other dermatoses listed as causes of the psoriasiform reaction pattern have been discussed in detail in other chapters. They are included again here for completeness, with a brief outline of the features which distinguish them from the other psoriasiform dermatoses.
This group of dermatoses is characterized, as a rule, by regular epidermal hyperplasia, although in the early stages such features are usually absent. Psoriasis, which is the prototype for this tissue reaction, will be considered first.
Psoriasis (psoriasis vulgaris) is a chronic, relapsing, papulosquamous dermatitis characterized by abnormal hyperproliferation of the epidermis. 2 It affects approximately 2% of the population and involves all racial groups, although it is uncommon in Africans, 3 South American Indians,4.5. and 6. and other indigenous people (Inuit, Aborigines, Ami). 7 Its incidence rate in a recent study from the United Kingdom was 14 per 10 000 person-years, 8 while a prevalence rate of just over 1% was recorded in a Spanish study. 9 Its incidence is high in Norway. 10
Psoriasis typically consists of well-circumscribed erythematous patches with a silvery white scale (plaque form). Characteristic bleeding points develop when the scale is removed. 11 This has been called Auspitz’s sign, although it appears that he has been wrongly credited with this observation. 12 Pruritus is sometimes present.13. and 14. There is a predilection for the extensor surfaces of the extremities, including the elbows and knees, and also the sacral region, scalp, 15 and nails.16. and 17. There is a broad spectrum of nail dystrophies associated with psoriasis, ranging from the common pitting, distal onycholysis, and loosening of the nail plate, to the less frequent discoloration and splinter hemorrhages seen in the nail bed. 18 Subungual hyperkeratosis may also develop. 19 Involvement of the palms and/or soles occurs in less than 20% of patients with psoriasis. 20 A scarring alopecia is rare.21. and 22. The lips are not commonly involved,23.24.25. and 26. and oral lesions in the form of whitish areas on the mucosa are quite rare. 27 Centrofacial involvement is a marker of severe disease.28. and 29. Penile lesions are more common in uncircumcised men. 30 When psoriasis involves the anogenital region of females, vulval scarring may ensue. 31 Lesions may develop at sites of trauma, and in peristomal skin. 32
In 5% or more of psoriatics, a seronegative polyarthritis develops.4. and 33. Controversy exists as to whether they represent two related but different disease processes. 34 A recent review highlighted their overlapping etiology and pathogenesis. 34 Achilles tendinitis is frequent in patients with psoriatic arthritis. 35 Psoriatic onycho-pachydermo-periostitis (POPP) is a rare subset of psoriatic arthritis. 36 Bilateral upper limb lymphedema has been reported in a patient with arthritis. 37 Psoriasis has also been reported in association with obesity, 38 vitiligo,39.40.41. and 42. gout, 43 diabetes,44.45. and 46. ankylosing spondylitis, ILVEN, 47 HIV infection, 48 benign migratory glossitis (geographic tongue),49. and 50. minor hair shaft abnormalities, 51 gliadin antibodies,52.53. and 54. and inflammatory bowel disease, particularly Crohn’s disease.33.55. and 56. Its association with bullous pemphigoid and other bullous diseases,57.58.59. and 60. perforating folliculitis, 61 lupus erythematosus,62. and 63. Kawasaki disease,64.65. and 66. hyper-IgE syndrome, 67 prolactinoma, 68 Vogt–Koyanagi–Harada syndrome, 69 insulinoma, 70 CD4+ lymphocytopenia, 71 Laurence–Moon–Biedl syndrome, 72 epidermal nevi, multiple exostoses, and surgical scars73 is probably a chance occurrence. There is a slight increase in the incidence of lymphoma and carcinoma of the larynx in patients with psoriasis, which is unrelated to mode of treatment.74.75. and 76. Psoriasiform eruptions may be a paraneoplastic phenomenon. 77 Heart disease appears to be increased in patients with psoriasis, as a consequence of increased atherosclerosis.45.78.79.80. and 81. This may be a consequence of significantly decreased levels of high-density lipoproteins.82. and 83. Serum leptin levels are increased in patients with psoriasis. 84 Patients show signs of insulin resistance. 85 Severe but not mild psoriasis is associated with an increased risk of death. 86
The mean age of onset of psoriasis is approximately 25 years, although it also develops sporadically in older persons, in whom it tends to have a milder course.87.88.89. and 90. Childhood cases are not uncommon,91.92.93. and 94. particularly in Scandinavia, where the disease commences in childhood in a high proportion of cases. 95 Plaque psoriasis is the most common type in childhood. 96 In those less than 2 years of age a psoriatic diaper rash with dissemination is the most common type. 96 Congenital onset is a rare occurrence. 97 A family history of psoriasis and an association with HLA-Cw6 are often present in those with early onset.94.98. and 99.
Facial involvement, nail involvement, and Koebner reactions are more common in early-onset psoriasis. 90 Psoriasis usually runs a chronic course, although spontaneous or treatment-induced remissions may occur. Its spontaneous clearance during the course of Kikuchi’s disease has been reported. 100 It can have a significant effect on the quality of life in those persons with the disease.101. and 102. Patients with palmoplantar psoriasis have more disability and discomfort than patients with other forms of psoriasis. 103 In order to assess the effects of treatment on psoriasis various indices of severity and area of involvement have been devised.104. and 105.
Several clinical variants of psoriasis have been recognized. Guttate psoriasis consists of 1–5 mm erythematous papules, which eventually develop a fine scale. It may be preceded by a streptococcal pharyngitis.106.107.108. and 109. Evidence of a preceding streptococcal infection is found in about two-thirds of cases of guttate psoriasis. 15 T lymphocytes specific for group A streptococcal antigens have been isolated from lesions of guttate psoriasis. 110 There is a predilection for the trunk and it is more common in children. 111 Clearing may occur spontaneously in weeks or months. 15 Psoriasis begins as the guttate form in 15% or more of cases. 99Erythrodermic psoriasis develops in approximately 2% of psoriatics and it accounts for 20% or more of erythrodermas.112.113.114. and 115. It is a severe form involving more than 90% of the skin with a high morbidity and an unpredictable course. 15 Erythrodermic psoriasis may be precipitated by administration of systemic steroids, by the excess use of topical steroids, by radiologic contrast media, 116 or by a preceding illness; it may develop as a complication of phototherapy. 112 Peripheral blood eosinophilia may be present in this form. 117Sebopsoriasis consists of yellowish-red, less well-marginated lesions, with variable degrees of scaling, often distributed in seborrheic regions of the body. 118 Rare clinical variants include a nevoid form,119. and 120. sometimes along the lines of Blaschko, 121 photosensitive psoriasis, 122 inverse (flexural) psoriasis, 123 follicular psoriasis,124. and 125. psoriasis spinulosa,111. and 126. psoriasis bullosa acquisita, 127 congenital erythrodermic psoriasis, 128 interdigital psoriasis, 129 rupioid psoriasis, 130 annular plaque-type psoriasis, 131 annular verrucous psoriasis, 132 verrucous (hypertrophic) psoriasis, 133 erythema gyratum repens-like psoriasis, 134 erythema annulare centrifugum-type psoriasis, 135 and linear psoriasis,62.136.137.138. and 139. although the occurrence of a linear form of psoriasis is not accepted by some authorities. Psoriasiform napkin dermatitis may also be a variant of psoriasis.96. and 140. Pustular psoriasis is regarded as a discrete entity. Different forms of the disease may alter their morphology and become a different clinical type. This phenotype switching may be a consequence of alterations in interleukin pathways. 141
Cases reported as psoriasiform acral dermatitis are now thought to represent a variant of psoriasis in children and not a discrete entity. This variant is characterized by cutaneous involvement of the digits without nail dystrophy. 142
There is a genetic proclivity to psoriasis, but no precise mode of inheritance is clear.87.143.144.145.146. and 147. The pattern is polygenetic148 rather than single-gene inheritance. A recessive mode of inheritance has been suggested in Swedish patients. 149 Concordance in monozygotic twins varies from 35% to 70% or more.87. and 150. It is 15–30% in dizygotic twins. 151 These statistics suggest that non-shared environmental influences also play a role. 152
Since 1994, many genetic loci (mainly on chromosomes 17q, 4q, 2p, 1q, and 6p) comprising at last nine genes have been under investigation.7.153.154.155. and 156. At least 19 different putative loci for genetic susceptibility to psoriasis have been reported.10. and 157. More recent studies suggest that there is a major susceptibility region for psoriasis on chromosome 6p21.3, near to HLA-C.158.159.160.161.162.163. and 164. It has been estimated that the proportion of genetic susceptibility attributable to this gene (PSORS1C3) is about 30%. 165 Attempts to link this gene to the CDSN gene (corneodesmosin), also near to HLA-C, were initially unsuccessful. 149 Both genes as well as the nearby HCR gene are now regarded as important psoriasis susceptibility genes in Chinese patients with psoriasis,166. and 167. although this has been questioned in a recent paper. 151 The CDSN gene is associated with psoriasis vulgaris in Caucasian but not in Japanese populations. 168 However, PSORS1 shows epistasis with genes at other locations, such as on 1p. 169PSORS1 contains several genes, some of which have an association with psoriasis. 170 Another study of psoriasis in Chinese has suggested that the MICA gene, another HLA-related gene on chromosome 6p21.3, may be a candidate gene. 171 The ACE gene variants may confer susceptibility in some populations.172. and 173. The leptin gene did not appear to be involved in a Turkish population. 174 Promoter region polymorphisms in the tumor necrosis factor-α gene have been associated with early-onset psoriasis in a Polish population, but not in Japanese, Chinese, or Korean people.175. and 176. In contrast, IL-12B gene polymorphisms, another cytokine gene, did confer a risk for psoriasis vulgaris in several different populations.176. and 177. Polymorphisms in the PTPN22 region are associated with psoriasis of early onset. 178 Other proinflammatory genes have also been implicated.179.180. and 181.
Psoriasis is associated with HLA-Cw6, B13 and B17 on serology,87.182.183.184. and 185. and more specifically with HLA-Cw*0602, HLA-DQA1*0104, and HLA-DRB1*0701 by PCR.161.186.187.188.189. and 190. An early study showed that all patients with guttate psoriasis carried the HLA-C allele compared with 20% of the control population. 191 This has not been confirmed by a subsequent study which concluded that the role of this allele in psoriasis has yet to be determined. 192 It has been suggested that PSORS1 may indeed be the HLA-CW*06 allele encoding the HLA-CW6 molecule. 7
MicroRNAs are implicated in the pathogenesis of psoriasis and also atopic eczema. Its effect may be mediated through a number of secondary pathways, including the TNF-α pathway. 193
Specific factors may trigger the onset or exacerbation of psoriasis. Trauma, infections,194. and 195. and drugs are accepted triggers, while the roles of climate, hormonal factors, cigarette smoking,8.196. and 197. alcohol, 197 mesotherapy, 198 internal malignancy,199. and 200. and stress201 are sometimes disputed. 87 Psoriasis may actually improve during pregnancy.202. and 203. It is often worse in the postpartum period.203. and 204. The development of lesions in response to trauma (Koebner reaction) is present in approximately one-third of cases.205. and 206. A link has been made between psoriasis and human papillomaviruses specifically associated with epidermodysplasia verruciformis, particularly the oncogenic HPV-5. 207 The prevalence of HPV in hairs plucked from patients with psoriasis is increased in patients treated with PUVA. 208 However, a more recent study did not find a specific causal role for HPV-5 or HPV-36 in the pathogenesis of psoriasis. 209 The role of Malassezia is more controversial. The improvement of scalp psoriasis treated with antifungal agents has suggested a role for Malassezia. M. restricta is the predominant species in psoriatic scale. M. globosa is also increased, but much less so. 210 Infection may precipitate guttate psoriasis.106. and 211. Various drugs may precipitate or exacerbate psoriasis,212. and 213. particularly lithium;214.215. and 216. other drugs include ecstasy, 217 quinidine, 218 clonidine, iodine, carbamazepine,219. and 220. olanzapine, 221 thalidomide, 222 terbinafine,223. and 224. indomethacin, 218 rofecoxib, 225 celecoxib, 225 various beta-blocker drugs,226. and 227. calcium channel blockers, 228 terfenadine, 229 urapidil (an α1-adrenergic blocker), 230 isotretinoin, interferon-α,196. and 231. interleukin-2, 232 the interleukin-1 receptor antagonist anakinra, 233 the TNF-α agonists adalimumab and infliximab,234.235.236.237.238. and 239. imiquimod,240.241.242. and 243. antimalarials and, rarely, the non-steroidal anti-inflammatory drugs and the angiotensin-converting enzyme (ACE) inhibitors. 244 However, a recent population-based case-control study found no association between the use of beta-blockers and psoriasis. 245 The drugs that precipitate/exacerbate psoriasis are listed in Table 4.1. It has been suggested that the eruptions produced by TNF-α agonists may be a ‘new model of adverse drug reaction’ rather than true psoriasis, as the histology shows lichenoid and spongiotic features. 246 The eruption triggered by efalizumab, a human anti-CD11a monoclonal antibody used in the treatment of psoriasis, consists of new papular lesions that arise in previously unaffected areas. 247 They usually do not necessitate termination of efalizumab therapy and may optionally be treated with corticosteroids. 247 A psoriasiform eruption, as opposed to true psoriasis, has been reported as a complication of several beta-blocker drugs, 248 and the related propafenone, 249 with fluorescein sodium used in angiography, 250 with the oral hypoglycemic agent glibenclamide, 251 with icodextrin, 252 and with terbinafine (see above). 253 The reactions caused by some of the beta-blocker drugs have a lichenoid histology despite their clinical appearance. Psoriasis has also followed the use of stem cell transplantation.254. and 255.
Psoriasis is a complex disease in which numerous abnormal findings have been reported.147. and 256. Despite this, the primary (initiating) alteration is unknown, but it appears that the molecular phenotype necessary for the clinical expression of psoriasis is present in all keratinocytes and includes a capacity for hyperproliferation and altered differentiation. Control of the expression of this phenotype involves the keratinocytes themselves, as well as cells of the immune system and various cytokines.257.258. and 259. Many of the changes in these elements may be epiphenomena or secondary and tertiary events in the pathogenetic cascade. As mentioned already, the primary alteration is not known, although it may involve the signal-transducing system of epidermal keratinocytes or the transcription regulatory elements associated with one or more cytokines.260.261. and 262. Stimulation of the immune system by superantigens has also been put forward as a primary event (see below). It is possible that different etiologies may initiate psoriasis in the genetically susceptible individual. Various aspects of the pathogenesis of psoriasis, particularly the immunopathogenesis, have recently been reviewed.170.263. and 264.
As the earliest detectable morphological change in psoriasis involves blood vessels in the papillary dermis, some research has focused on their role in the pathogenetic cascade.265.266. and 267. Vascular changes in psoriasis include dilatation and tortuosity of vessels in the papillary dermis, as well as angiogenesis (neovascularization) 268 and the formation of high endothelial venules, which are specialized postcapillary venules lined by tall columnar or cuboidal endothelial cells. These factors are important in expanding the size of the microcirculation which may, in turn, facilitate the trafficking of T lymphocytes, of the Th1 subclass, into the skin, 269 thus maintaining the psoriatic plaque. 270 Blood flow is increased in these plaques. 271 The high endothelial venules play an important role in the cutaneous recruitment of circulating lymphocytes. 272 Microvascular hyperpermeability is another feature of severe psoriasis. This appears to be mediated by circulating vascular endothelial growth factor (VEGF). 273 Angiogenesis is stimulated by factors such as interleukin-8 and transforming growth factor-α (TGF-α).265. and 274. The presence of angiogenesis in psoriasis has been challenged. Using three-dimensional reconstructions, it has been suggested that downgrowths of the rete ridges include the vessels of the horizontal plexus giving the appearance of intrapapillary capillaries. 275 This study has not been confirmed. In short, it appears that dermal capillary changes alone are unlikely to be causal in psoriasis.267. and 270.
Recruitment of lymphocytes to the papillary dermis is an important factor. 276 This is aided by various chemoattractants such as platelet-activating factor and leukotriene B4. 277 Some of these lymphocytes are already activated before entering the skin, while still circulating in the bloodstream. 278 The lymphocytes bind to endothelial cells in venules in the papillary dermis as a consequence of the enhanced expression of various adhesion molecules by endothelial cells. 279 It appears that lymphocyte function-associated antigen type 1 (LFA-1), consisting of CD11a and CD18 subunits, 280 which acts as a ligand for intercellular adhesion molecule-1 (ICAM-1), and ICAM-1 itself play a major role in the adhesion of CD4+ T cells to endothelial cells. 281 Furthermore, TNF-α may play an important role in induction of adhesion molecules on endothelial cells. 281 Vascular adhesion protein-1 (VAP-1) is also overexpressed in psoriasis. 282 Efalizumab, an anti-CD11a antibody, has been used to treat psoriasis (see below). Lymphocytes then diapedese transendothelially and pass through the vessel wall into the papillary dermis. Neutrophils will subsequently leave the vessels in a similar way and migrate into the stratum corneum. 283 Chemotactic factors such as C5a anaphylatoxin are important in their recruitment. 284
While the importance of T lymphocytes in the pathogenesis of psoriasis is accepted,285.286. and 287. there has been some dispute over the relative importance of CD4+ and CD8+ lymphocytes. 288 The T cells in lesional dermis are predominantly CD4+. Cells migrating into the epidermis are mostly CD8+.170.289. and 290. Which type produces keratinocyte proliferation by the release of mediators (cytokines) is still disputed. 291 Both now appear to be involved, but CD8+ cells are particularly important.170.292.293. and 294. CD8+ cells in the epidermis express the Vβ T-cell receptor subgroups Vβ3 and Vβ13.1. 295 Another study has shown an increase in the Vβ2 receptor in skin-homing lymphocytes in psoriasis. 296 In psoriasis CD4+CD25+ regulatory T cells are functionally deficient in suppressing effector T-cell proliferation. 297
As mentioned above, leukocytes bind to endothelial cells in the papillary dermis, prior to their passage from the vessels. This process is under the control of adhesion molecules, which can be classified into three distinct groups:
1. the immunoglobulin gene superfamily which includes ICAM-1 (CD54) and ICAM-2 and VCAM-1 (vascular cell adhesion molecule-1)
2. integrins298
3. selectins (the most important of which is E-selectin).
One or more of these adhesion molecules lead to the selective adhesion of CD4, CD45RO helper T cells. 277 Other categories of adhesion regulators, such as the proline-directed serine/threonine kinases, of which CDK5 is a member, exist. They appear to influence cadherins and integrins. 299 The expression of CDK5 is reduced in psoriasis. 299 Various cytokines appear to induce the enhanced expression of these adhesion molecules in psoriasis; they include interleukin-1 (IL-1), interleukin 2 (IL-2), TNF-α, interferon-γ (IFN-γ), and interleukin-4 (IL-4).276. and 300. On the other hand, ultraviolet-B radiation reduces the adhesive interactions and expression of adhesion molecules, possibly explaining its mode of action in the treatment of psoriasis. 301 Serum levels of soluble E-selectin correlate with the extent of psoriatic lesions. 302
There is a complex interplay between the various cytokines found in the skin in psoriasis; some cytokines have more than one action. They are produced mostly by lymphocytes, although keratinocytes release at least two.258.303. and 304. Dendritic cells and macrophages also produce important cytokines. One of these is interleukin-12 (IL-12) which induces differentiation of naïve CD4 T lymphocytes to T-helper 1 (Th1) cells, which are key effector cells in the pathogenesis of psoriasis as a consequence of their production of various cytokines such as IFN-γ and IL-2.305. and 306. It also activates natural killer cells. 305 IL-23, which is closely related to IL-12 in structure, stimulates a subset of CD4+ lymphocytes to produce IL-17, which induces the production of further proinflammatory cytokines, predominantly by endothelial cells and macrophages.305. and 307. Therapy using an IL-12/IL-23 antibody is currently undergoing clinical trials (see below). 305 Interleukin-18 (IL-18), a novel cytokine produced mainly by monocytes and macrophages but also synthesized by keratinocytes, plays an important role in the Th1 response by stimulating the production of IFN-γ and TNF. It is increased in the serum of patients with psoriasis. 308 In contrast, low levels of IL-10, an anti-inflammatory cytokine, have been found in psoriatic lesions.309. and 310. The many functions of these various cytokines include stimulation of keratinocytes, 311 vascular changes (see above), control of lymphocyte trafficking (see above), and stimulation of neutrophil chemotaxis. Exacerbations of psoriasis are preceded by a rapid increase in neutrophil chemotaxis. Interleukin-8 (IL-8) is a cytokine with possibly more chemotactic activity than the various complement factors.312.313. and 314. Another is psoriasin (S100A7), a protein belonging to the calcium-binding S100 family. It is a potent inflammatory mediator. 315 Two other members of this family, S100A8 and S100A9, are increased in psoriasis and contribute to the hyperproliferation of psoriatic skin. 316 The importance of the cytokines in the pathogenesis is shown by the down-regulatory effects of cyclosporine (ciclosporin) on cytokines and cytokine receptors in the treatment of psoriasis.317. and 318. The role of the various cytokines in psoriasis has been reviewed. 319 They are summarized in Table 4.2.
The final pathway in the pathogenesis of psoriasis involves the stimulation of keratinocytes by factors such as TNF-α, IL-6, IL-8, TGF-α, IFN-γ, granulocyte/macrophage colony stimulating factor, and the phospholipase C/protein kinase C signal transduction system (see below). IFN-γ, which plays an important role in the growth stimulation of keratinocyte stem cells in psoriasis, can be produced by mast cells as well as lymphocytes. 320 TNF-α also has a major role. Its release from cells is under the influence of TNF-α-converting enzyme (TACE). 321 The success of the various TNF-α neutralizing modalities in the treatment of psoriasis has led to a re-evaluation of the role of TNF-α in the pathogenesis of psoriasis. It seems likely that dysregulation of innate immunity, involving natural killer (NK)-T cells, plays a role in the pathogenesis of psoriasis. 322 This is supported by the finding of increased levels of perforin, the cytotoxic product of NK cells, in the epidermis of psoriatic plaques. 323 Paradoxically, circulating NK cells are reduced in psoriasis. 324 There is also an overexpression of the CXC chemokines, interleukin-8 (IL-8) and GRO/melanoma growth-stimulatory activity (GRO-α). 325 They are potent activators of neutrophils and lymphocytes but also stimulate proliferation of keratinocytes. 325 These factors produce an alteration in the turnover time for the epidermis: 3–4 days in psoriasis compared with the usual 13 days in normal skin. 326 It has been estimated that there is a 12-fold increase in the number of basal and suprabasal keratinocytes in cell cycling. 327 TGF-α, which is elevated in psoriasis, is predominantly synthesized in subcorneal keratinocytes. 328 It is a potent mitogen that can also stimulate angiogenesis. In contrast, TGF-β has an inhibitory effect on epithelial cell proliferation. Down-regulation of its receptor in psoriatic epidermis has the effect of diminishing this inhibitory influence. 329 Amphiregulin, a cytokine which acts as an epidermal growth factor, is also increased in psoriatic keratinocytes. 330 Transgenic mice engineered to overproduce amphiregulin develop a psoriasis-like phenotype, suggesting that a genetically transmitted alteration of amphiregulin synthesis may be a possible cause of the cascade of events in psoriasis. 319 There is increased activation of the Src-family of tyrosine kinases (SFKs) in psoriasis.331. and 332. They are important regulators of epidermal growth and differentiation. 331 The existence of an increased number of epidermal growth factor receptors (EGF-R), resulting from their persistence at all levels of the epidermis instead of just the basal layer, may be just as important.327. and 333. Antigen-presenting cells expressing the common heat shock protein receptor CD91 have been found juxtaposed to keratinocytes expressing HSP70, a ligand for CD91 in a mouse model of psoriasis.334. and 335. These activated antigen-presenting cells produce TNF-α in close proximity to these keratinocytes. 334 T-cadherin, E-cadherin, P-cadherin, and protein kinase D expression all seem to play a part in the regulation of epidermal growth in psoriasis.336.337. and 338. Associated with this hyperproliferation of keratinocytes is a mild increase in apoptosis, and a reduction in the number of Bcl-2-positive cells in the basal layer.339.340.341. and 342. Bcl-2 expression in lymphocytes is increased. 343 Suppression of apoptosis also occurs in psoriasis. 344 Survivin, a member of the inhibitor of apoptosis protein (IAP) family, is increased in psoriasis. It appears to be regulated by the transcriptional factor NF-κB. 345 Some of the therapies for psoriasis act by their increase in apoptosis. 346 A senescence switch involving p16 may prevent malignant transformation of this up-regulated epidermis. 347 Also methylation of the p16INK4a gene promoter is found in psoriatic epidermis. 348
As a consequence of the hyperproliferation of keratinocytes, there is enhanced expression of keratins K6, K16, and K17, 349 and reduced amounts of the keratins indicative of differentiation (K1, K2, and K10). K16 is also expressed in non-lesional psoriatic skin and may serve as a marker of preclinical psoriasis. 350 It has been found recently that altered peptide ligands derived from keratin 17 are capable of inhibiting proliferative responses of psoriatic T cells and keratinocyte proliferation in vitro. 351 This opens up another therapeutic option in the treatment of psoriasis. 351 There is a unique subpopulation of cells in psoriatic epidermis that coexpress K6 and K10. 352 There is also increased nuclear β-catenin in suprabasal cells, 353 alterations in cell-surface glycoconjugates, 354 and variations in the epidermal differentiation complex, a cluster of genes on chromosome 1q21 that fulfill important functions in the terminal differentiation in the human epidermis. 355 Tight junction components are also altered. 356
The role of microbiological superantigens in the pathogenesis of psoriasis is gaining acceptance, although formal proof of a pathogenic role is still lacking.357.358.359.360.361. and 362. Superantigens are toxins of microbial origin that not only stimulate certain classes of T cells363 but also have the capability to interact directly (without prior processing) with MHC class II molecules: this leads to considerable T-cell activation and cytokine release. 364 Streptococcal antigens can function as superantigens and it is suggested that they may act as the initiating factor in some cases of guttate psoriasis, in part through superantigen-driven generation of Vβ-restricted CLA-positive skin homing lymphocytes.365.366. and 367. A recent study has confirmed that prior pharyngeal infection is a risk factor for guttate psoriasis. 368 Peripheral blood lymphocytes from patients with psoriasis are generally hyporesponsive to streptococcal superantigens, 369 but there is a subpopulation of CD4+ cells that produces interferon-γ (IFN-γ) in response to this antigen.370. and 371. Superantigens produced by Staphylococcus aureus may also be triggering factors.372. and 373.Malassezia furfur is also capable of exacerbating psoriasis. 374 Patients with psoriasis harbor human papillomavirus type 5 (HPV-5) in a significant number of cases. 375 Antibodies to HPV-5, one of the types associated with epidermodysplasia verruciformis, appear to be generated in the epidermal repair process, but whether they contribute to a proliferation of keratinocytes in psoriasis is not known. 375 The high prevalence of cytomegalovirus antigenemia in psoriasis is possibly related to reactivation of the virus by elevated levels of tumor necrosis factor-α (TNF-α).376. and 377. Endogenous retroviral sequences are expressed in psoriasis. 378 They are part of the normal human genome. Their possible role in the pathogenesis of psoriasis is currently being investigated.378. and 379. It has been suggested recently that a broad range of viral and bacterial stimuli may stimulate psoriasis, not by acting on T cells directly but by stimulating plasmacytoid dendritic cells (myeloid-derived cells) to produce large amounts of type 1 interferons (IFN α/β).34. and 380. These disparate findings remain to be integrated into a unitarian theory of pathogenesis.
Other findings in psoriasis that may play some role in the pathogenetic cascade include the increased expression of heat shock proteins by keratinocytes,381.382.383. and 384. an increase in reactive oxygen species, 385 excessive activation of a phospholipase C/protein kinase C signal transduction system which stimulates keratinocyte proliferation,261.386. and 387. overexpression of serpin squamous cell carcinoma antigens in psoriatic skin, 388 and increased lysophosphatidyl choline activity in lesional skin. This substance is a lysophospholipid which is chemotactic for monocytes and stimulates the expression of certain adhesion molecules – VCAM-1 and ICAM-1. 389 The finding of immunoreactants in the stratum corneum and the dermis is not thought to be of major pathogenetic significance. 390 There is an up-regulation of the gap junction protein connexin 26 between keratinocytes of psoriasis. 391 There is also an overexpression of matrix metalloproteinases 2 (MMP-2) and 9 (MMP-9) and other related members of the ADAM family.392.393. and 394. Increased levels of kallikreins are also found in the stratum corneum and serum of patients with psoriasis. 395 Telomerase activity is increased in peripheral blood mononuclear cells in psoriasis. The level correlates with disease severity. 396 There is also increased expression of the natural killer cell inhibitory receptor CD94/NKG2A and CD158b on circulating and lesional T cells in psoriasis and this elevation correlates with disease severity. 397 The significance of these findings is uncertain. Increased levels of elafin, also termed skin-derived anti-leukoproteinase (SKALP), are found in subcorneal keratinocytes of psoriatic lesions. 398 It is a potent elastase inhibitor which may protect the epidermis from the proteolytic activity of neutrophils. 398 The epidermis is also protected against bacterial infections by the release of granulysin by lesional T cells and dendrocytes. 399 The exact role of neuropeptides (including substance P) remains to be clarified. They provide a possible explanation for the triggering action of stress in the exacerbation of psoriasis. 400 Increased serum cortisol levels are another possible mechanism by which stress influences psoriasis. 401 Serotonin levels are also increased in the lesions of psoriasis. 402
In concluding this section, it should not be forgotten that psoriasis is characterized by erythematosquamous lesions. The erythematous nature of the lesions results from the dilatation and increase in vessels in the dermal papillae, which have a thin, overlying layer of epidermal keratinocytes. The clinical thickening of the lesions results from the psoriasiform epidermal hyperplasia brought about by increased mitotic activity in basal keratinocytes through the action of various cytokines with growth factor activity. The scale is composed of parakeratotic cells, resulting from increased transit time, and a focal admixture of neutrophils. 403 All of these features are possibly the consequence of an autoreactive inflammatory process mediated by T lymphocytes of the Th1 subclass.263. and 318. This cytokine profile may be the result of local factors and not determined by a specific genotype. 404
In assessing the effects of treatment, there is a need to use appropriate, reproducible assessment criteria that can be compared with the results of other trials. The current benchmark is the PASI (Psoriasis Area and Severity Index), and reductions of 75% or more of this Index are regarded as a suitable response to a particular therapy. It has recently been suggested that a 50% reduction in the PASI is a meaningful response to any therapy. 405 Patient compliance is suboptimal in the treatment of psoriasis as treatments are generally prolonged and may have side effects. Up to 40% of patients at any point in time are not receiving any treatment for their condition. 406 A similar number do not use medications as directed. 407 Treatment also varies between different dermatologists. About 40% of patients receive topical therapy alone. 408 Clinical trials of various treatment schedules usually have a placebo group if they are not of a comparative nature. A study of these placebo groups has found that they generally do poorly, as might be expected. 409
The various therapies used in psoriasis target different components of the pathogenetic cascade, although some have an effect on more than one component. 410
In the management of guttate psoriasis, there is little evidence of the efficacy of the standard treatment of this disease – tar and UVB phototherapy. 411 Dithranol and topical steroids with or without UVB phototherapy have also been used. The vitamin D3 analogue calcipotriol is sometimes effective. 411 There is no evidence that antistreptococcal interventions are effective in the management of guttate or chronic plaque psoriasis, 412 but tonsillectomy may be worthwhile in patients with recurrent streptococcal infections that seem to trigger or maintain their skin disease. 413
Topical corticosteroids remain the most commonly prescribed therapy for psoriasis, but they are frequently used in tandem with other agents.414. and 415. The use of anthralin and tar has declined with the increased availability of topical calcipotriol and tazarotene. 414 A recent consensus conference on topical therapies for psoriasis concluded that there were inadequate studies to draw any conclusions regarding the efficacy, safety, and tolerability of tar and anthralin (dithranol). 415 Calcipotriol ointment gives the same quality of life improvements as dithranol. 416 Calcipotriol has proved effective when compared to all other topical treatments of psoriasis. 416 It is safe provided no more than 100 g of formulation is used each week. 416 When used with methylprednisolone it produces decreased p53 and Ki-67 expression in treated skin. 417 A recent trial found that calcipotriene plus betamethasone dipropionate was more effective than either of the individual components alone. 418 Calcitriol ointment may be used with the calcineurin inhibitor tacrolimus for psoriasis in sensitive areas such as the face and intertriginous areas. 419 The two therapies have also been used to treat recalcitrant acrodermatitis continua of Hallopeau. 420 Tacrolimus and calcipotriol were found to be equally effective when compared with each other. 421 Another trial found that tacrolimus was more effective than calcitriol. 419 Topical calcitriol appears to target several different pathways in the pathogenetic cascade, producing decreased keratinocyte proliferation, normalized differentiation of keratinocytes, and decreased immune activation. It also normalizes the expression of adhesion molecules.422.423. and 424. Pimecrolimus has been used with narrowband UVB to treat childhood psoriasis. 425 Zinc pyrithione is another topical therapy that can be used. 426 It produces apoptosis in treated keratinocytes.
A similar mechanism is thought to be responsible for the involution of psoriatic hyperplasia that follows PUVA, and narrowband UVB.427.428. and 429. They also reduce the numbers of lymphocytes, macrophages and dendritic cells in treated skin, but PUVA is the only modality that decreases epidermal Langerhans cells. 430 Phototherapy also induces the enhanced expression of insulin-like growth factor-binding protein-7. 431 Phototherapy remains the most commonly used therapy for widespread psoriasis but its use is declining because of its association with cutaneous malignancies. In chronic plaque psoriasis, PUVA achieves clearance in more patients with fewer treatment sessions and longer remissions than narrowband UVB. 432 The latter therapy causes a reduction/depletion in epidermal CD4+ cells.433. and 434. It may rarely cause a subepidermal blister. 429 A new UVB source generated by a 308 nm excimer laser produces significant T-cell depletion in the skin, increased apoptosis of keratinocytes and decreased Ki-67.435. and 436. Results of this therapy seem promising. Results are enhanced by applying topical methoxypsoralen cream prior to the phototherapy. 437 The pulsed dye laser has limited use; it has met with some success. 438
Balneophototherapy, involving the use of saltwater baths followed by UVB irradiation, 439 is an attempt to reproduce the beneficial effects of Dead Sea climatotherapy.440. and 441. This therapy has all the potential complications of ultraviolet exposure. It has been found that oral retinoids, when used with PUVA therapy, reduce the risk of squamous cell carcinoma but not basal cell carcinoma. 442 Not unexpectedly, the melanocortin 1 receptor (MC1R) genotype influences the erythemal sensitivity of skin to PUVA. 443
A wide range of systemic medications are available for the treatment of psoriasis. The three most commonly used are retinoids, methotrexate, and cyclosporine. 444 They are approved by the FDA. Before discussing these systemic therapies, it should be emphasized that topical treatments will probably remain the mainstay of psoriasis therapy for most patients. 157 Care should also be exercised in the use of various therapies in pregnant patients. This subject was reviewed recently. 445
Retinoids’ mechanism of action is by reducing the proliferation of keratinocytes, as well as having an inhibitory effect on tissue inflammation.446. and 447. They have also been used to treat verrucous psoriasis. 448 Systemic retinoids combined with biological agents (see below) offer a promising method of managing refractory psoriasis. 380
Methotrexate is an effective therapy for moderate to severe psoriasis,449.450. and 451. although there is a need to improve its safety profile by adhering to protocols for its use. 452 It down-regulates the expression of some adhesion molecules and this may contribute to its therapeutic effects. 449 Long-term use of methotrexate is often discontinued because of its toxic effects, or the loss of a response to treatment. 453 Combining etanercept with methotrexate is reasonable when etanercept monotherapy produces insufficient improvement. 454 Vasculitis is a very rare complication of its use. 455
Cyclosporine is more effective than methotrexate from a short-term perspective. 456 It is safe in low doses. It affects several different steps in the cascade. Not only does it interfere with the expression of keratinocyte adhesion molecules, 457 but it also normalizes the basal lamina and the overexpression of cyclins.458.459. and 460. It decreases the number of dermal dendrocytes461 and inhibits IL-12 production by monocytes. 462 There is an improved pattern of CK14 expression, and CK10 recovers to normal levels. 463
Other systemic drugs available include tacrolimus, mycophenolate mofetil, hydroxyurea, 6-thioguanine,464. and 465. and sulfasalazine. 444
Treatment options for severe psoriasis are either time-consuming (e.g. phototherapy) or have the potential for organ toxicity with chronic use. This applies to methotrexate, retinoids, and cyclosporine. 466 The newer immune-based (biological) therapies are becoming widely used for moderate to severe psoriasis, but they are expensive.467.468.469.470.471.472. and 473. Patient satisfaction is high. 474 The tumor necrosis factor-α (TNF-α) antagonists have proved more effective than efalizumab, a CD11a blocker. 472 Etanercept is usually favored over the other TNF-α antagonists, 472 although increasing evidence suggests that infliximab is more efficacious and better tolerated (see below). Guidelines for the use of biological agents in the treatment of psoriasis have recently been published (2008). 475
TNF-α antagonists block an important inflammatory cytokine. TNF-α is increased as a consequence of the overexpression of proinflammatory type 1 (Th1) cytokines. 476 TNF-α up-regulates the expression of CTACK/CCL27, a cutaneous T-cell attracting chemokine. 477 As a consequence there is a decrease in skin-homing CD4+ and CD8+ lymphocytes. TNF-α antagonists impair dendritic cell function478 and decrease epidermal Langerhans cells. 479 They produce apoptosis of keratinocytes. 480 Infliximab down-regulates the inhibitor of apoptosis, survivin, in keratinocytes and endothelial cells. 481 There are three main groups of TNF-α antagonists:
1. Entanercept – a recombinant TNF-α receptor fused to the Fc fragment of IgG1; it is administered subcutaneously.
2. Infliximab – a mouse/human chimeric monoclonal antibody to TNF-α; it is administered intravenously and requires hospitalization. 482
TNF-α antagonists are all effective in the treatment of psoriasis and psoriatic arthritis.485.486.487.488.489.490.491. and 492. Sometimes dosage schedules need to be altered for an individual patient to control the disease or side effects of the therapy. 493 Some patients develop a flare of their psoriasis with the development of new lesions despite continued treatment.476.494.495. and 496. Other cases may flare on cessation of treatment,476. and 496. or recur several months after treatment has ceased.497.498. and 499. They may be used in combination with systemic and topical medications when they confer an additional benefit. 476 One such combination that has been trialed is etanercept and acitretin. 500 The use of TNF-α antagonists allows tapering of methotrexate. 501 They are better than methotrexate or a placebo, 502 and improve quality of life.503. and 504. A recent meta-analysis of published trials found that infliximab is the most efficacious agent for moderate-to-severe psoriasis, followed by adalimumab. 505 Another meta-analysis also found that infliximab was the most efficacious biological agent followed by etanercept, efalizumab, and alefacept. 506 Infliximab is also effective in treating psoriatic nail disease. 507 TNF-α antagonists may produce weight gain. 508 Patients should be screened for tuberculosis prior to commencing therapy because they suppress immunity.509. and 510. A recent evidence-based assessment concluded that there is no strong evidence to support the performance of screening and monitoring tests when using systemic biological agents, despite earlier recommendations that they be carried out. 511 There may also be a need to consider available vaccinations. 512 Dose schedules may need to be altered in cases of obesity. 513 Finally, mention should be made of a rare complication, demyelination. 514
Monoclonal antibody to CD11a (efalizumab). Although this drug is not considered to be as effective as the TNF-α antagonists, several trials of this drug have produced good results.280.515.516.517.518. and 519. It is given by subcutaneous injection. 476 There may be a rebound of psoriasis when the drug is ceased but this has been prevented by the use of cyclosporine. 476 Several patients who have been refractory to efalizumab have developed erythrodermic flares when switched to etanercept. 520 The drug interferes with T-cell activation and migration to the skin.521. and 522.
Alefacept reduces the number of memory-effector (CD45RO+) T cells.409.523. and 524. It has proved effective in several clinical trials.525. and 526. Alefacept has been used specifically for scalp psoriasis527 and palmoplantar psoriasis. 528 Another drug designed to reduce the number of pathogenic T cells, an anti-CD4 antibody, has not produced significant improvement.529. and 530.
Newer treatments. Immune therapies based on new concepts of disease pathogenesis are now being trialed.531. and 532. One of these drugs, ilodecakin, is a recombinant IL-10 which aims to down-regulate Th1 responses which are overexpressed in psoriasis. Only temporary improvement was achieved in one trial. 533 Further trials are needed with a recombinant IL-11. 476 ABT-874, an interleukin-12/23 monoclonal antibody, now undergoing phase II trials, has been highly effective and well tolerated.305. and 534.
Newer non-immune therapies include botulinum toxin-type A, used to treat a small number of patients with flexural psoriasis, 123 oral bexarotene, a synthetic retinoid X receptor, 535 paclitaxel, 536 everolimus, a rapamycin-derived macrolide, 537 the thiazolidinediones, used in the treatment of type 2 diabetes, and a new generation of all-trans retinoic acid metabolism blocking agents (RAMBA). In this latter group is an oral, anti-proliferative agent, known as R115866 (Rambazole); it shows promise. 538 Finally, circumin (the active component of the Indian spice turmeric) has been used as a complementary therapy for psoriasis, but in a formal study there was only a low response. 466
Psoriasis is a dynamic process and consequently the histopathological changes vary during the evolution and subsequent resolution of individual lesions. The earliest changes, seen in lesions of less than 24 hours’ duration, consist of dilatation and congestion of vessels in the papillary dermis and a mild, perivascular, lymphocytic infiltrate, with some adjacent edema. There is also some exocytosis of lymphocytes into the epidermis overlying the vessels and this is usually associated with mild spongiosis (Fig. 4.1). The epidermis is otherwise normal. This is soon followed by the formation of mounds of parakeratosis, with exocytosis of neutrophils through the epidermis to reach the summits of these parakeratotic foci. 543 There is often overlying orthokeratosis of normal basket-weave type and loss of the underlying granular layer. At this papular stage, increased mitotic activity can be seen in the basal layer of the epidermis associated with a modest amount of psoriasiform acanthosis (Fig. 4.2). Keratinocytes in the upper epidermis show some cytoplasmic pallor. Blood vessels in the papillary dermis are still dilated and somewhat tortuous, and their lumen may contain neutrophils. Lymphatic channels are also increased. 544 Very few neutrophils are ever present in the perivascular infiltrate: this consists mainly of lymphocytes, Langerhans cells, and indeterminate cells. 545 A few extravasated erythrocytes may also be present. These changes can also be seen in guttate psoriasis although the epidermal hyperplasia is usually mild in this variant of psoriasis. 2
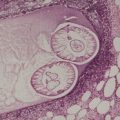
Psoriasis. (A) A very early lesion with dilated vessels in a dermal papilla, perivascular cuffing with lymphocytes, and exocytosis of lymphocytes and a neutrophil or two. (B) A slightly later stage with neutrophils migrating to the summits of the parakeratotic mounds. (C) Dilated vessels are in the papillary dermis. (H & E)
Psoriasis. Mitoses are evident in keratinocytes within the epidermis. (H & E)
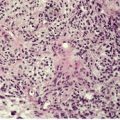
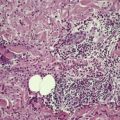
In early plaques of psoriasis and in ‘hot spots’ of more established plaques, 546 there are mounds of parakeratosis containing neutrophils, which usually migrate to the upper layers (summits) of these mounds (Fig. 4.3). With time, confluent parakeratosis develops (Fig. 4.4). Several layers of parakeratosis containing neutrophils, with intervening layers of orthokeratosis, are sometimes present. While intracorneal collections of neutrophils (Munro microabscesses) are common, similar collections in the spinous layer (spongiform pustules of Kogoj) are less so. They are also much smaller than in pustular psoriasis. These pustules contain lymphocytes in addition to neutrophils. The epidermis now shows psoriasiform (regular) hyperplasia, with relatively thin suprapapillary plates overlying the dilated vessels of the papillary dermis (Fig. 4.5A). Ki-67 expression is increased. 547 A few mononuclear cells are usually present in the lower layers of the suprapapillary epidermis. The dermal inflammatory cell infiltrate is usually a little heavier than in earlier lesions. It includes activated T lymphocytes, 548 fewer Langerhans cells than in earlier lesions, and very occasional neutrophils. 545 A subset of spindle-shaped macrophages, situated along the basement membrane, has been described as a characteristic feature. These so-called ‘lining cells’ are positive for CD11c. Plasma cells and eosinophils are usually absent, 541 but eosinophil cationic protein has been identified, particularly in the upper third of the epidermis in psoriasis. 549 Plasma cells may be present in patients with HIV infection. 550
Psoriasis. (A) There is psoriasiform hyperplasia of the epidermis. (B) Neutrophils are present in the upper layers of the overlying parakeratotic scale – neutrophils migrating to the ‘summits’ of the parakeratotic mounds. (H & E)
Psoriasis. Confluent parakeratosis overlies an epidermis showing psoriasiform hyperplasia. (H & E)
Psoriasis. (A) The dilated vessels in the papillary dermis are well shown. The suprapapillary epidermis (‘plate’) is relatively thin. (B) There is some coalescence of the tips of the rete pegs. (H & E)
With time, there may be club-shaped thickening of the lower rete pegs with coalescence of these in some areas (Fig. 4.5B).540. and 541. Later lesions show orthokeratosis, an intact granular layer, and some thickening of the suprapapillary plates. Exocytosis of inflammatory cells is usually mild. The finding of numerous fatty vacuoles in the papillary dermis – pseudolipomatosis cutis (see p. 848) – is of doubtful significance.551. and 552. The phenomenon has not been satisfactorily explained. 552 Differentiation of late lesions of psoriasis from lichen simplex chronicus may be difficult, although in the latter condition the suprapapillary plates and granular layer are usually more prominent and there may be vertically oriented collagen bundles in the papillary dermis. 2 If psoriatic plaques are rubbed or scratched, the histopathological features of the underlying psoriasis may be obscured by these superimposed changes.
The term ‘psoriatic neurodermatitis’ has been proposed for pruritic, lichenified plaques on the elbows and/or knees. 553 Lesions were more numerous, smaller, more keratotic, and less excoriated than in typical lichen simplex chronicus. Microscopically the lesions showed microabscesses in the horny layer, hypogranulosis, regular acanthosis, and thinning of the suprapapillary plates. 553 It is thought that these cases represent psoriasis with superimposed lichen simplex chronicus. 553
In resolving or treated plaques of psoriasis there is a progressive diminution in the inflammatory infiltrate, a reduction in the amount of epidermal hyperplasia, and restoration of the granular layer. 539 Vessels in the papillary dermis are still dilated, although by now there is an increase in fibroblasts in this region with mild fibrosis. 539 Only after 10–14 weeks of treatment do the histological appearances return to normal. 554
Minor changes which have been reported in psoriasis of the scalp include sebaceous gland atrophy, a decrease in hair follicle size, and thinner hair shafts.555. and 556. Other features of scalp psoriasis include dilatation of infundibula with parakeratosis at the lips of the infundibular ostia, papillomatosis, and scattered apoptotic keratinocytes. 556 Munro microabscesses are said to be uncommon in this region. 541 Another regional variation is the lessened epidermal hyperplasia in psoriasis of the penis and vulva; 541 spongiosis may be present.
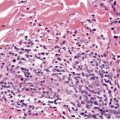
Spongiosis has already been mentioned as a feature of the early lesions of psoriasis, and of psoriasis occurring in various regions such as the hands and feet and genital regions. It may also occur in erythrodermic psoriasis (see below). Ackerman has also drawn attention to its presence in other situations (see p. 119). The author has now seen several cases that caused diagnostic confusion, initially because of significant spongiosis, but which, over time, have evolved into classic psoriasis. Their initial biopsies showed spongiosis, mounds of parakeratosis containing neutrophils, dilated vessels in the papillary dermis, and a mild, superficial perivascular infiltrate of lymphocytes. The term spongiotic psoriasis is an appropriate designation for these cases (Fig. 4.6).
Psoriasis. There is mild spongiosis at the tips of the rete pegs. (H & E)
The nail plate in nail psoriasis shows hyperkeratosis, focal parakeratosis, and variable neutrophil exocytosis into the parakeratotic layer. Spongiosis is a common feature of nail psoriasis. 19 Examination of PAS-stained sections is necessary before making a diagnosis of nail psoriasis because onychomycosis and psoriasis may show similar histology. 19
In erythrodermic psoriasis, the appearances may resemble those described in early lesions of psoriasis, possibly a reflection of the early medical intervention that usually occurs in this condition. 557 Dilatation of superficial vessels is usually quite prominent. A cornified layer is usually absent. Sometimes the histological changes do not resemble those of psoriasis at all.
In follicular psoriasis, there is follicular plugging with marked parakeratosis in the mid-zone of the ostium. 124 The dermal inflammatory infiltrate is both perivascular and perifollicular.
In annular verrucous psoriasis, there is exaggerated papillomatosis resulting in finger-like projections of the epidermis. 132 Similar changes have been reported in cases called ‘verrucous psoriasis’. The papillomatosis and bowing of the peripheral rete ridges toward the center of the lesion mimic the appearances of verruca vulgaris. 133
Skin tumors have developed at sites treated with PUVA therapy,558. and 559. particularly after prolonged exposure.74.560. and 561. Prolonged UVB therapy results in the accumulation of DNA photoproducts in the cells, although adaptive responses occur. 562 The use of coal tar does not produce any appreciable increase in skin cancers. 563 Variants of seborrheic keratosis have also been reported in psoriatic patients receiving treatment with ultraviolet radiation. 564 Of relevance is the controversy over whether patients with psoriasis have an inherently low risk of developing skin cancer,565. and 566. although recent studies suggest that this is not so.567.568. and 569. Psoriasis may protect against the development of actinic keratoses.566. and 570. Another rare complication of treatment is cutaneous ulceration, which has been reported following methotrexate therapy. 571
The histopathological differentiation of psoriasis from chronic eczematous dermatitis, particularly seborrheic dermatitis, is sometimes difficult. 572 The presence of prominent spongiosis involving the rete ridges generally rules out psoriasis, while the presence of a leukocytic exudate, other than in a folliculocentric distribution, is quite uncommon in seborrheic dermatitis. 1 In seborrheic dermatitis there is often less epidermal hyperplasia than in psoriasis. Spongiotic psoriasis (see above) can be very difficult to distinguish from other spongiotic processes. The presence of mounds of parakeratosis containing neutrophils and a dermal infiltrate that is usually mild are clues to the diagnosis of psoriasis.
Distinguishing palmoplantar psoriasis from an eczematous dermatitis at these sites can be difficult, because they share some histological features. A recent study found that the presence of multiple parakeratotic foci, placed vertically, and alternating with orthohyperkeratosis favored a diagnosis of psoriasis. 573 Psoriasis in this site often shows spongiosis but it is often restricted to the lower epidermis. Spongiotic vesicles were present in approximately 75% of cases in this series. 573 Neutrophils and/or plasma are often present in the parakeratotic layers in palmoplantar psoriasis but it is very uncommon for the neutrophils to be at the summits of the parakeratotic mounds. 573
The features that distinguish psoriasis from lichen simplex chronicus have been discussed above. Spongiosis is sometimes present in the rete ridges in this latter condition if it is superimposed on an eczematous process. Psoriatic neurodermatitis needs consideration (see above). 553
In pityriasis rubra pilaris, there is mild to moderate psoriasiform hyperplasia, parakeratotic lipping of follicles and, in some lesions, alternating zones of orthokeratosis and parakeratosis in both horizontal and vertical directions.
Pustular psoriasis has more prominent spongiform pustulation than psoriasis vulgaris, particularly at the shoulders of the lesions.
There are numerous cytoplasmic organelles in the keratinocytes, reflecting their hyperactivity. These are decreased with treatment. 575 Tonofilaments and desmosomes are reduced in number and size and there is also a reduction in the number of keratohyaline granules. 574 Vessels in the papillary dermis are dilated with abundant fenestrations. 574 Neutrophils are said to be polar in shape with ruffled cell membranes. 576
Psoriasiform keratosis is the name given by Walsh, Hurt, and Santa Cruz to lesions that closely mimic psoriasis in the same way that the term lichenoid keratosis is used for solitary lesions with a resemblance to lichen planus. 577 Psoriasiform keratoses are usually solitary lesions on the extremities of elderly persons who have no other clinical features of psoriasis at the time of presentation, or on subsequent follow-up examination.577. and 578. Other sites of involvement have been the scalp, neck, shoulders, and back. 577 Clinically, the lesions resemble a seborrheic keratosis, basal cell carcinoma, actinic keratosis, or Bowen’s disease. A diagnosis of psoriasis is only occasionally made.
The lesions are usually well-defined, scaly plaques which vary in size from 0.5 to 3 cm in diameter.
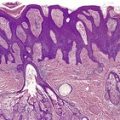
The microscopic features resemble psoriasis, but with more parakeratosis and less psoriasiform hyperplasia (for the amount of parakeratosis) than is usual in most cases of chronic psoriasis (Fig. 4.7). This imparts a vague resemblance to a seborrheic keratosis. The parakeratosis is often diffuse, but may be focal. 578 There are intracorneal collections of neutrophils, often arranged in vertical tiers. 577 There may be mild spongiosis.
Psoriasiform keratosis. There is a close resemblance to psoriasis but this was a solitary lesion on the leg of an elderly male with no history of psoriasis. The parakeratosis is sometimes prominent.
The dermis shows a mild to moderate superficial perivascular infiltrate of lymphocytes. Some vascular proliferation is often present, but this may be a reflection of the anatomic site (lower legs) of many lesions.
The PAS stain is negative for fungal organisms.
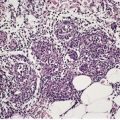
Psoriasis, seborrheic dermatitis and cases with overlap features may occur in patients infected with the human immunodeficiency virus (HIV).579.580.581. and 582. The term ‘AIDS-associated psoriasiform dermatitis’ has been used, particularly for those cases with features of both conditions. Interestingly, there is often more uniformity in the histopathological expression than in the clinical presentations. In some circumstances, onset of the disease, or its exacerbation, has been associated with the initial seroconversion. In others, exacerbations are associated with cutaneous or systemic infections. The severity of the disease is variable. A Reiter’s syndrome-like pattern has been seen in this condition. 583
The epidermis shows psoriasiform hyperplasia but, unlike psoriasis, there is no thinning of the suprapapillary plate. There are scattered apoptotic keratinocytes within the epidermis, usually associated with some lymphocyte exocytosis. Perivascular lymphocytes in the dermis may show karyorrhexis, giving rise to small amounts of nuclear dust. Plasma cells are often present in small numbers. 550
Pustular psoriasis is a rare, acute form of psoriasiform dermatosis characterized by the widespread eruption of numerous sterile pustules on an erythematous base and associated with constitutional symptoms.113.584.585. and 586. Skin tenderness, a neutrophil leukocytosis, and an absolute lymphopenia587 may precede the onset of the pustules. These may continue to develop in waves for several weeks or longer before remitting. Arthritis, 588 generalized erythroderma, 588 hypocalcemia,589. and 590. and lesions of the mucous membranes, including fissured tongue and benign migratory glossitis (geographical tongue), may develop in the course of the disease. 591 Amyloidosis, 592 acute respiratory distress syndrome,593.594. and 595. and a bullous disorder596 are extremely rare complications. Erythema gyratum repens has developed in resolving pustular psoriasis. 597
Several clinical variants of pustular psoriasis are recognized.584. and 588. The Von Zumbusch type (generalized pustular psoriasis) is the most common variant. It has an explosive onset and a mortality as high as 30% in some of the earlier series. Impetigo herpetiformis is a controversial entity defined by some on the basis of flexural involvement with centripetal spread of the pustules and by others as a variant of pustular psoriasis occurring in pregnancy.598.599.600.601. and 602. As such, it is a rare pruritic dermatosis of pregnancy; fewer than 150 cases have been reported. 603 Onset in pregnancy is usually in the third trimester, although it develops earlier in subsequent pregnancies.604.605. and 606. It usually remits postpartum, but may flare with the use of oral contraceptives. 607 Menstrual exacerbations that occurred for 7 years postpartum have been reported in one patient. 608 Fetal mortality is high as a consequence of placental insufficiency. 607 A subset related to hypoparathyroidism with hypocalcemia is sometimes included in impetigo herpetiformis.609.610. and 611. Hyperparathyroidism was present in one case. 612 Impetigo herpetiformis has been followed by generalized pustular psoriasis, suggesting that it is part of the pustular psoriasis spectrum and not a distinct entity. 613 The acral variant of pustular psoriasis arises in a setting of acrodermatitis continua, which is a localized pustular eruption of one or more digits with displacement and dystrophy of the nails.584.614.615.616. and 617. It has been caused by oral terbinafine. 618 The development of generalized pustular psoriasis in acrodermatitis continua has a bad prognosis.584. and 614. Palmoplantar pustulosis is associated with plaque psoriasis in about 20% of cases. It is sufficiently distinct in its clinical, genetic, and biological features to be regarded as a separate entity, distinct from psoriasis (see p. 133).15.619. and 620. Other variants include an exanthematic form,584.614. and 621.diaper pustular psoriasis, 622 an annular variant623.624. and 625. with some resemblance to subcorneal pustular dermatosis, a linear variant,626. and 627. and a localized form which consists of pustular psoriasis developing in pre-existing plaques of psoriasis.584. and 588. The annular variant is the most common form of pustular psoriasis in children. 625 Some of the cases reported in the past as exanthematous variants may represent examples of acute generalized exanthematous pustulosis (see p. 151). 628 A case of pustular psoriasis limited to the penis has been reported. 629 There was no pre-existing condition. Pustular psoriasis developing over keloids may be an example of the Koebner phenomenon. 630
Generalized pustular psoriasis may develop in three main clinical settings. 584 In the first group there is a long history of psoriasis of early onset. In these cases the pustular psoriasis is often precipitated by some external provocative agent (see below). In the second group, there is preceding psoriasis of atypical form in which the onset was relatively late in life. Precipitating factors are not usually present. In the third group, pustular psoriasis arises without pre-existing psoriasis. Pustular psoriasis may rarely develop as a consequence of persistent pustulosis of the palms and soles. Familial cases of pustular psoriasis631. and 632. and onset in childhood have also been reported.631.632.633.634. and 635. In children, pustular psoriasis can be complicated by sterile, lytic lesions of bones.634. and 636. The development of renal failure and cholestatic jaundice in one patient may have been a coincidence. 637
Numerous factors have been implicated in precipitating pustular psoriasis. 638 These include infections, sunlight, burns, 639 ultraviolet radiation from tanning salon use, 624 alcohol, malignancy, metabolic and endocrine factors, pregnancy, 640 emotional stress, and drugs. The drugs include lithium, 641 iodides, clopidogrel, 642 non-steroidal anti-inflammatory agents including phenylbutazone, 643 and aceclofenac, 644 beta-blockers, 645 penicillin and related drugs, 638 procaine, cyclosporine646. and 647. and following its withdrawal after short-term use, 648 infliximab649 and other tumor necrosis factor inhibitors, 650 oral terbinafine,651. and 652. bupropion, 653 prednisolone, 654 sulfonamides, doxorubicin, 655 morphine, hydroxychloroquine, progesterone, 656 nystatin, and topical calcipotriol.657. and 658. The withdrawal of steroids is a common precipitating factor which may be included in this category. These various agents that may precipitate pustular psoriasis are listed in Table 4.3. Generalized pustular psoriasis has developed in patients with bullous and non-bullous ichthyosiform erythroderma. 659
One of the most striking features of pustular psoriasis is the enhanced chemotaxis of neutrophils, which is even more marked than in psoriasis.660. and 661. The chemotactic factors in the affected areas of skin include leukotrienes, complement products, and cathepsin 1. 662
The treatment options for pustular psoriasis include phototherapy, photochemotherapy, retinoids, and immunosuppressive therapy. 663 Isotretinoin is comparable to etretinate in its effectiveness. Acitretin and narrowband UVB phototherapy have also been used. 664 It should be remembered that retinoids are teratogens and their use during pregnancy is contraindicated. Infliximab and etanercept can be used in refractory cases.665.666. and 667. In impetigo herpetiformis, cyclosporine has produced good results. 668 This drug is associated with a higher risk of premature rupture of the membranes, but it is not thought to be teratogenic. 669 Cessation of cyclosporine has produced flares of the usual type of pustular psoriasis. 670 Often impetigo herpetiformis does not respond to oral corticosteroids. 603 Narrowband UVB and PUVA phototherapy, methotrexate, and oral retinoids have all been used. 671Acrodermatitis continua is usually recalcitrant to treatment. 672 Topical treatments have included corticosteroids, tar, dithranol, fluorouracil, calcipotriol, and topical tacrolimus 0.1% ointment. 673 Systemic therapy, with or without topical therapy, has included retinoids, methotrexate, cyclosporine, PUVA, colchicine, dapsone, corticosteroids, and oral propylthiouracil combined with methotrexate.668. and 673.
The diagnostic feature is the presence of intraepidermal pustules at various stages of development (Fig. 4.8). In early lesions, the epidermis is usually only slightly acanthotic, while psoriasiform hyperplasia is seen only in older and persistent lesions (Fig. 4.9). Mitoses are usually present within the epidermis. Neutrophils migrate from dilated vessels in the papillary dermis into the epidermis. They aggregate beneath the stratum corneum and in the upper malpighian layer between degenerate and thinned keratinocytes to form the so-called ‘spongiform pustules of Kogoj’ (Fig. 4.10). 676 The subcorneal pustules have a thin roof of stratum corneum. In later lesions these are replaced by scale crusts with collections of neutrophils trapped between parakeratotic layers. A few eosinophils may be present in the infiltrate.
Early pustular psoriasis. There is a heavy infiltrate of neutrophils in the upper layers of the epidermis and beneath the stratum corneum. (H & E)
Pustular psoriasis (old lesion). There is pronounced psoriasiform hyperplasia of the epidermis and spongiform pustulation in the upper layers. (H & E)
Pustular psoriasis. A spongiform pustule of Kogoj is shown. (H & E)
The blood vessels in the papillary dermis are usually dilated and there is a perivascular infiltrate of lymphocytes and a few neutrophils. Large mononuclear cells were noted in the pustules and in the dermis in one report of impetigo herpetiformis. 677 They were thought to be specific for this variant of pustular psoriasis, although they were specifically excluded in a subsequent report of this condition. 677
Multipolypoid herniations of basal keratinocytes have been described protruding into the dermis through large gaps in the basal lamina. Neutrophil proteases are probably responsible for this change. In another study there were gaps between the endothelial cells of dermal blood vessels. 678
Reiter’s syndrome is usually defined as the triad of non-gonococcal urethritis, ocular inflammation and arthritis. 679 The presence of mucocutaneous lesions is sometimes included as a fourth feature. 680 Reiter’s syndrome occurs in approximately 30% of patients with reactive arthritis, which in turn develops in 1–3% of patients with sexually acquired, non-gonococcal infections of the genital tract.679. and 681. Reiter’s syndrome has also been associated with certain bacterial gut infections, including those due to Shigella flexneri, Yersinia enterocolitica, 682 and, rarely, Campylobacter jejuni. 683 The genital infectious agent which is usually incriminated is Chlamydia trachomatis, but Ureaplasma urealyticum and species of Mycoplasma have also been isolated.679. and 683. Chlamydial elementary bodies have been detected by immunofluorescence and monoclonal antibodies in the synovium of patients with reactive arthritis, which to date has always been sterile by conventional cultures. 684Chlamydia-specific antigens have been detected in a biopsy of the cutaneous lesions of Reiter’s syndrome. 685 Reiter’s syndrome has also been induced by systemic interferon-α treatment, 686 and by adalimumab in combination with leflunomide. 687
There is genetic susceptibility to the development of reactive arthritis and Reiter’s syndrome and this is manifest by the presence of the histocompatibility antigen HLA-B27.682. and 688. Other clinical features of Reiter’s syndrome include a marked preponderance in males, a mean age of onset in the third decade of life, and a variable, often relapsing course.688.689. and 690. Some cases begin in childhood. 691 An association with the acquired immunodeficiency syndrome has been reported.692. and 693.
The mucocutaneous lesions, already alluded to, include a circinate balanitis with perimeatal erosions and mucosal ulcers. 694 An ulcerative vulvitis is rarely present.695. and 696. In 10–30% of cases there are crusted erythematous papules and plaques with a predilection for the soles of the feet, genitalia, perineum, buttocks, scalp, and extensor surfaces of the extremities. 690 Some lesions may be frankly pustular, resembling pustular psoriasis. These cutaneous lesions are known by the name ‘keratoderma blennorrhagica’. They usually heal after several weeks, without scarring. Nail changes can also occur. 697 Ackerman believes that Reiter’s syndrome is a variant of psoriasis. 698
The use of the eponym ‘Reiter’s syndrome’ is declining in use in the medical literature, possibly because Reiter, a physician leader of the Nazi party, authorized medical experiments on prisoners of concentration camps. 699 Nevertheless, it was used in a recent major review of the subject. 700
In most biopsies, the cutaneous lesions of Reiter’s syndrome are indistinguishable from pustular psoriasis. Accordingly, there is psoriasiform epidermal hyperplasia with a thick horny layer (Fig. 4.11). This is most prominent in lesions on the palms and soles and least prominent in penile and buccal lesions. Spongiform pustulation with exocytosis of neutrophils is another conspicuous feature. 701 A variable inflammatory cell infiltrate, usually including a few neutrophils, is present in the upper dermis. 702 A mild, leukocytoclastic vasculitis has been observed in the papillary dermis of several cases. 685
Reiter’s syndrome. The appearances may be indistinguishable from pustular psoriasis. (H & E)
Various histological features have been claimed to be more suggestive of Reiter’s syndrome than pustular psoriasis. These include a thicker horny layer, larger spongiform pustules, eczematous changes, a thicker suprapapillary plate of epidermis, the presence of neutrophils in the dermis, and the absence of clubbing of the rete ridges. The horny layer is sometimes more loosely attached than in pustular psoriasis, leading to its partial detachment during processing of the specimen. These various features are usually not sufficiently different from the findings in pustular psoriasis to allow a confident distinction to be made between the two conditions on biopsy material.
Pityriasis rubra pilaris (PRP) is a rare, erythematosquamous dermatosis of unknown etiology with a prevalence that varies from 1 in 5000 to 1 in 50 000 in various populations. 703 It is characterized by small follicular papules with a central keratin plug, perifollicular erythema with a tendency to become confluent but with islands of sparing, palmoplantar keratoderma, often with edema, and pityriasis capitis.704.705.706.707. and 708. The condition often begins with a seborrheic dermatitis-like rash on the face or scalp which rapidly spreads downwards.709.710.711.712. and 713. In other patients, particularly juveniles, the disease starts on the lower half of the body.714. and 715. Some patients may become erythrodermic.716.717.718. and 719. Exacerbation of PRP with ultraviolet exposure is well recognized; much less common is its presentation in a photoexposed distribution. 720 A variable degree of pruritus is often present. 721 Cases with localized lesions, restricted often to the elbows and knees, occur. 722 The thigh is another site for localized disease. 723 Severe forms of PRP have been reported in patients infected with the human immunodeficiency virus (HIV).724.725.726.727. and 728. Cystic acne may coexist. 725 Arthropathy and osteoporosis have been present in another patient with PRP. 729
Pityriasis rubra pilaris has been reported as the initial manifestation of internal neoplasia.730. and 731. Its association with Down syndrome may be fortuitous. 732 Rarely, dermatomyositis may present with a PRP-like eruption. 733 A rare familial form with suggested autosomal dominant inheritance occurs. 734
Nail changes,704. and 735. alopecia,705. and 706. and, rarely, multiple seborrheic keratoses or cutaneous malignancies may occur in patients with pityriasis rubra pilaris.736. and 737. Hypothyroidism is a rare association. 738 The age of onset and clinical course are quite variable.739.740.741. and 742. For this reason, the clinical classification into five types, as proposed by Griffiths, has not been used here. 743 Remission occurs within 6 months to 2 years in about 50% of cases,703.744. and 745. but recurrences occur in more than 10% of cases.741. and 746.
Although there is some resemblance to vitamin A deficiency (phrynoderma), serum vitamin A levels are normal. 716 Reduced levels of retinol-binding protein (the specific carrier of vitamin A) have been reported, 747 but the results of this work have not been confirmed.748. and 749. Epidermal cell kinetics show an increased rate of cell proliferation.750. and 751. It has been suggested that the acute juvenile form of PRP is a superantigen-mediated disease. 712
Topical retinoids, either tazarotene gel or tretinoin 0.05% cream,723. and 752. and topical corticosteroids742 are the treatment of choice for localized disease. Topical calcipotriol and tar have also been used. Oral retinoids may be beneficial for more widespread disease.703.732.741. and 753. The side effects of oral retinoids can warrant discontinuation of this therapy in children. 703 Other treatment modalities that have been used include vitamin D analogues, cyclosporine, 713 methotrexate, azathioprine, and narrowband UVB. Intravenous immunoglobulin, 754 infliximab,753.755. and 756. extracorporeal photochemotherapy, 719 and etanercept alone or in combination with oral acitretin757 have been used for cases refractory to other treatments. Etanercept is not currently approved by the FDA for this purpose. 757
The changes are most marked when erythema is greatest, and least impressive in biopsies of follicular papules. 707 There is diffuse orthokeratosis with spotted parakeratosis which also forms a collarette around the follicular ostia (Fig. 4.12). Some follicular plugging is often present (Fig. 4.13). 759 Parakeratosis is not prominent in early lesions. These changes may be stated in another way – alternating orthokeratosis and parakeratosis in both vertical and horizontal directions (Fig. 4.14). 758 However, many cases will be missed if this criterion is too rigidly applied (Fig. 4.15). There is also acanthosis: this is never as regular as that seen in psoriasis. There are broad rete ridges and thick suprapapillary plates. Hypergranulosis is often prominent and this may be focal or confluent. 758 An unusual perinuclear vacuolization is sometimes seen in cells in the malpighian layer and there may be some vacuolar change involving the pilary outer root sheath. Spongiosis, usually mild, is present in about 10% of cases.753. and 760. There is a superficial perivascular and perifollicular lymphocytic infiltrate in the dermis. While the infiltrate is usually mild, a heavy infiltrate, which is rarely lichenoid in distribution, is sometimes present. 761 Eosinophils and plasma cells are occasionally present in the infiltrate. 762 Folliculitis is a rare complication. 740 Another rare histological finding is the presence of focal acantholytic dyskeratosis in the lesions of pityriasis rubra pilaris.737.763.764.765. and 766. Epidermolytic hyperkeratosis has been reported in one case. 762
Pityriasis rubra pilaris. (A) As it should be, with psoriasiform hyperplasia and overlying ‘geometric’ parakeratosis. (B) High power view of the case shown in (A). (C) There is a very thick parakeratotic and orthokeratotic scale. Not too many conditions give such a thick scale. (H & E)
Pityriasis rubra pilaris. There is mild keratotic follicular plugging and lipping. (H & E)
Pityriasis rubra pilaris. (A) There is alternating orthokeratosis and parakeratosis in both a horizontal and a vertical direction. (B) and (C) High power view of other cases of this condition. (H & E)
Pityriasis rubra pilaris.The diagnosis of cases like this can be difficult. The author misses more cases of this condition (on the initial biopsy) than any other. (H & E)
In contrast to pityriasis rubra pilaris, vitamin A deficiency shows no focal parakeratosis, irregular acanthosis, or dermal inflammatory infiltrate. 705
The term ‘parapsoriasis’, as originally introduced, referred to a heterogeneous group of asymptomatic, scaly dermatoses with some clinical resemblance to psoriasis.768.769. and 770. These conditions were further characterized by chronicity and resistance to therapy. Three distinct entities are now recognized as having been included in the original concept of ‘parapsoriasis’ – pityriasis lichenoides, chronic superficial dermatitis (small plaque parapsoriasis, digitate dermatosis), and large plaque parapsoriasis (atrophic parapsoriasis, retiform parapsoriasis, patch-stage mycosis fungoides). 769
Confusion has arisen because of the retention of the term ‘parapsoriasis’ for two distinct conditions. In the United States, the term ‘parapsoriasis en plaque’ is usually used to refer to the entity that in the United Kingdom is called chronic superficial dermatitis. 771 The term ‘parapsoriasis’ is also used for a condition with large plaques, which in 10–30% of cases progresses to a frank T-cell lymphoma of the skin. 772 Studies have indicated monoclonal populations of T cells in 20% or more of cases of large plaque parapsoriasis. 773
Brief mention will be made of the three entities included in the original concept of parapsoriasis.
Pityriasis lichenoides shows features of both a chronic lymphocytic vasculitis and the lichenoid tissue reaction (see p. 230). It should no longer be considered as a variant of parapsoriasis.
Chronic superficial dermatitis (small plaque parapsoriasis) resembles a ‘mild eczema’ and it is therefore discussed in detail on page 119, as part of the spongiotic tissue reaction. The spongiosis is often quite mild and in chronic lesions may be absent. In these circumstances, the epidermal acanthosis may assume psoriasiform proportions, hence its mention here also. It differs from psoriasis by the absence of dilated vessels in the papillary dermis and the absence of neutrophil exocytosis. Furthermore, chronic superficial dermatitis lacks a thin suprapapillary plate and there is a paucity of mitoses in the keratinocytes (Fig. 4.16). Lymphocytes with a normal mature morphology are often found in the papillary dermis in chronic superficial dermatitis. This feature, combined with the regular acanthosis and focal parakeratosis, allows a diagnosis to be made in many cases with the scanning power of the light microscope. A dominant clonal pattern of T cells has been identified in some cases.774. and 775. Accordingly, it is often regarded as an early stage of cutaneous T-cell lymphoma. A recent study of 28 cases of small plaque parapsoriasis found one case that presented later with plaque-stage mycosis fungoides. The other 27 cases were non-progressive although three of these cases had an oligoclonal pattern on molecular genetic studies. 776
Chronic superficial dermatitis with psoriasiform hyperplasia, thick suprapapillary ‘plates’ of epidermis, and a mild, superficial lymphocytic infiltrate with some upward spread. (H & E)
Large plaque parapsoriasis, the third entity included originally as ‘parapsoriasis’, may also show features of psoriasiform epidermal hyperplasia, although in atrophic and poikilodermatous lesions the epidermis is thin with loss of the rete ridge pattern. Basal vacuolar change and epidermotropism of lymphocytes are usually present. Large plaque parapsoriasis, a stage in the evolution of mycosis fungoides, is considered with other cutaneous lymphoid infiltrates on page 975.
Finally, brief mention will be made of the term ‘guttate parapsoriasis’. This term has been used in the past synonymously with both pityriasis lichenoides and chronic superficial dermatitis. It is best avoided. 770
Lichen simplex chronicus (‘circumscribed neurodermatitis’) is an idiopathic disorder in which scaly, thickened plaques develop in response to persistent rubbing of pruritic sites.777. and 778. There is a predilection for the nape of the neck, the ulnar border of the forearms, the wrists, the pretibial region, the dorsa of the feet, and the perianal and genital region.778.779. and 780. Atopic individuals are more prone than others to develop lichen simplex chronicus. 781
Although not psoriasiform, mention is made of a recently described entity that clinically resembles lichen simplex chronicus or lichen amyloidosus but which has no histological similarities at all – pretibial pruritic papular dermatitis. 782 The authors of that paper proposed that it was a response to chronic rubbing, possibly with other contributing factors such as xerosis, contact with irritants, and emotional distress. The lesions were red to flesh colored, pruritic papules 3–8 mm in diameter. There was a cobblestone appearance in some later lesions. Lesions were unilateral in 33/44 cases and bilateral in 11/44. The histology showed mild compact orthokeratosis, flattening of the rete ridges, superficial dermal fibrosis, and a mild to moderate superficial and mid-dermal infiltrate of lymphocytes, histiocytes and a few eosinophils. Stellate cells were present, probably a reflection of scratching. The published photomicrographs show some resemblance to pigmented purpuric dermatosis but there was no hemosiderin. 782
Kinetic studies in lichen simplex chronicus have shown epidermal cell proliferation similar to that seen in psoriasis although the transit time of the cells is not as fast. 779 There is also an increase in mitochondrial enzymes in keratinocytes and in the number of melanocytes in the basal layer. 783 Although these kinetic aspects are known, there is still no explanation for the pathogenesis of these plaques and the underlying pruritus; it is apparent, however, that self-induced trauma plays an important localizing role. 779
Although beyond the scope of this book an oral variant of lichen simplex chronicus has recently been described. 784 Also known as benign alveolar ridge keratosis, it is a common lesion that presents as a white papule or plaque on the keratinized gingiva of the maxillary or mandibular alveolar ridge. It is probably traumatic/frictional in origin. 784
The treatment of lichen simplex chronicus is often difficult, unless the scratching habit can be stopped. A sedative antihistamine may be useful. The usual treatment is a topical corticosteroid but if thick plaques are present, potent corticosteroids under occlusion or triamcinolone injections may be needed.
A thick layer of compact orthokeratosis (resembling that seen on normal palms and soles) is present, overlying hypergranulosis. Focal zones of parakeratosis are sometimes interspersed with the orthokeratosis, but there is not the confluent parakeratosis of psoriasis. 779 The epidermis shows psoriasiform hyperplasia with thicker rete ridges of less even length than in psoriasis. Epidermal thickness and volume are greater than in psoriasis. 779 Minimal papillomatosis is sometimes present in a few areas. Focal excoriation is another change that may be seen in lichen simplex chronicus.
There is marked thickening of the papillary dermis with bundles of collagen arranged in vertical streaks (Fig. 4.17). 785 Scattered inflammatory cells and some fibroblasts are usually present in this region of the dermis.
Lichen simplex chronicus. The rete pegs are thinner than usual but the vertical ‘streaking’ of collagen in the papillary dermis is well developed. (H & E)
Regional variations occur in lichen simplex chronicus. Epidermal hyperplasia is usually quite mild in lesions on the lip, while vertical-streaked collagen is unusual in lesions on the scalp or in mucocutaneous regions such as the vulva and perianal area. 785
Changes like those of lichen simplex chronicus may be superimposed on other dermatoses such as lichen planus (hypertrophic lichen planus), mycosis fungoides, actinic reticuloid, and eczematous dermatitides including atopic dermatitis.778. and 781. These changes are particularly prominent in some solar keratoses of the hands or forearms.
The term ‘prurigo nodularis’ (see p. 670) is used for lesions with a nodular clinical appearance and prominent epidermal hyperplasia of pseudoepitheliomatous rather than psoriasiform type. At times, lesions with overlapping clinical and histopathological features of both lichen simplex chronicus and prurigo nodularis are found. Prurigo nodularis is one condition in which the histological picture often lags behind the clinical appearance. The author has seen numerous cases, regarded by experienced dermatologists as prurigo nodularis, in which the histological picture was that of lichen simplex chronicus.
This group of other psoriasiform dermatoses has been arbitrarily separated from the so-called ‘major psoriasiform dermatoses’ because of their inclusion in various other chapters, on the basis of their etiology or of other histopathological features. The comparative histopathological features of these various psoriasiform diseases are shown in Table 4.4.
The various ‘eczematous’ dermatitides (allergic contact dermatitis, seborrheic dermatitis, nummular dermatitis, and atopic dermatitis) may show prominent psoriasiform epidermal hyperplasia in their subacute and chronic stages.
In subacute lesions, spongiosis is usually sufficiently obvious to allow a correct diagnosis. In some chronic lesions, particularly if activity has been dampened by treatment prior to the taking of a biopsy, the spongiosis may be quite mild or even absent. The features which distinguish chronic seborrheic dermatitis from psoriasis have been discussed on page 80. In some cases of chronic atopic and nummular dermatitis the epidermal hyperplasia is not as regular and as even as that seen in psoriasis, although this is by no means invariable. The presence of eosinophils and plasma cells in the superficial dermis would tend to exclude psoriasis. They may be found in any of the chronic spongiotic dermatitides that may simulate psoriasis histopathologically. The changes of lichen simplex chronicus may be superimposed on these chronic spongiotic dermatitides (Fig. 4.18).
Chronic allergic contact dermatitis with psoriasiform hyperplasia of the epidermis and small foci of spongiosis. (H & E)
Erythroderma (exfoliative dermatitis) is a cutaneous reaction pattern characterized by erythema, edema and scaling of all or most of the skin surface, often accompanied by pruritus (see p. 507). It may complicate a pre-existing dermatosis, follow the ingestion of a drug or be associated with an internal cancer or with cutaneous T-cell lymphoma.
The findings are variable and often non-specific. Psoriasiform hyperplasia, sometimes accompanied by mild spongiosis, may be present in cases of erythroderma not thought to be of psoriatic origin, while presumptive cases of erythrodermic psoriasis may show only non-specific changes in the epidermis. The difficulties encountered in an attempted histopathological diagnosis of erythroderma are mentioned on page 508.
Mycosis fungoides is a cutaneous T-cell lymphoma with three clinical stages – patch, plaque, and tumor. Its varied clinical features are discussed on page 973.
Psoriasiform hyperplasia of the epidermis is not uncommon in mycosis fungoides. It is usually of mild to moderate proportions. The presence of epidermotropism and variable cytological atypia of the lymphocytic infiltrate are features which distinguish this condition from other psoriasiform dermatoses (Fig. 4.19).
Mycosis fungoides. There is psoriasiform hyperplasia of the epidermis and conspicuous epidermotropism of lymphocytes. (H & E)
Psoriasiform epidermal hyperplasia may be present in lesions of chronic candidosis (see p. 589) and, rarely, in chronic dermatophyte infections, most notably in tinea imbricata.
The rete ridges are not unusually long in the psoriasiform hyperplasia of chronic candidosis. There are usually a few neutrophils and some serum in the overlying parakeratotic scale. Fungal elements, in the form of yeasts and pseudohyphae, may be sparse and difficult to find with the PAS stain. They are often more readily seen in methenamine silver preparations.
Hyphae and spores are usually abundant in the thick stratum corneum in tinea imbricata.
The acronym ILVEN is often used in place of the more cumbersome inflammatory linear verrucous epidermal nevus. This condition is a variant of epidermal nevus which usually presents as a pruritic, linear eruption on the lower extremities (see p. 669). It must be distinguished from linear psoriasis.
The characteristic feature is the presence of alternating zones of orthokeratosis and parakeratosis in a horizontal direction, overlying a psoriasiform epidermis (Fig. 4.20). The zones of parakeratosis overlie areas of agranulosis. Focal mild spongiosis is often present as well.
Inflammatory linear verrucous epidermal nevus (ILVEN). (A) Note the psoriasiform epidermal hyperplasia. (B) There are broad zones of parakeratosis alternating with orthokeratosis. The granular layer is absent beneath the parakeratotic zones. (H & E)
Norwegian (crusted) scabies is a rare form of scabies which is usually found in the mentally and physically debilitated; it also occurs in immunosuppressed individuals (see p. 656). There are widespread crusted and secondarily infected hyperkeratotic lesions.
Overlying the psoriasiform epidermis there is a very thick layer of orthokeratosis and parakeratosis containing numerous scabies mites at all stages of development. The appearances are characteristic (Fig. 4.21).
Norwegian scabies. The thick stratum corneum which overlies the psoriasiform epidermis contains a number of scabies mites. (H & E)
There is a variant of Bowen’s disease in which the epidermis shows psoriasiform hyperplasia (see p. 679). It has no distinguishing clinical features.
There is psoriasiform hyperplasia with a thick suprapapillary plate. Atypical keratinocytes usually involve the full thickness of the epidermis; sometimes there is sparing of the basal layer and the acrosyringium (Fig. 4.22). Mitoses and dyskeratotic cells are usually present. Uncommonly, the psoriasiform variant of Bowen’s disease is composed of pale pagetoid cells.
Bowen’s disease. There is psoriasiform epidermal hyperplasia and full thickness atypia. (H & E)
The clear (pale) cell acanthoma presents as a papulonodular lesion, usually on the lower parts of the legs (see p. 674).
The characteristic feature is the presence of a well-demarcated area of psoriasiform epidermal hyperplasia in which the cells have palely staining cytoplasm. Exocytosis of inflammatory cells may also be present. The pale keratinocytes contain abundant glycogen.
This is a rare, severe, autosomal recessive form of ichthyosis (see p. 251). It is usually manifest at birth.
There is prominent orthokeratosis and focal parakeratosis overlying a normal or thickened granular layer. Psoriasiform epidermal hyperplasia is sometimes present, although usually the epidermis shows only moderate acanthosis. Psoriasiform hyperplasia is also found in some cases of ichthyosis congenita (see p. 251) and Netherton’s syndrome (see p. 253). Biopsies of the ichthyosis linearis circumflexa that is a component of Netherton’s syndrome may be misdiagnosed as congenital psoriasis (Fig. 4.23).
Ichthyosis linearis circumflexa. Distinguishing this lesion from psoriasis is sometimes difficult. (H & E)
The ‘herald patch’ of pityriasis rosea may show the psoriasiform tissue reaction (see p. 101).
There is usually acanthosis and only mild psoriasiform hyperplasia. Small ‘Pautrier simulants’ (pityriasiform spongiosis), composed of inflammatory cells in a spongiotic focus, are often seen. There is usually focal parakeratosis overlying the epidermis.
Pellagra is caused by an inadequate amount of niacin (nicotinic acid) in the tissues. Skin lesions include a scaly erythematous rash in sun-exposed areas, sometimes with blistering, followed by hyperpigmentation and epithelial desquamation (see p. 483).
The findings are not usually diagnostic. Sometimes there is psoriasiform acanthosis with pallor of the upper epidermis and overlying orthokeratosis and focal parakeratosis. The psoriasiform acanthosis is more common in mixed nutritional deficiency states.
Acrodermatitis enteropathica, a rare disorder resulting from zinc deficiency, presents with periorificial and acral lesions which may be eczematous, vesiculobullous, pustular, or an admixture of these patterns (see p. 488).
In established lesions there is confluent parakeratosis overlying psoriasiform epidermal hyperplasia. The upper layers of the epidermis show a characteristic pallor and sometimes there is focal necrosis or subcorneal clefting. The epidermal pallor disappears in late lesions.
Necrolytic migratory erythema is the term used for the cutaneous lesions of the glucagonoma syndrome. This syndrome in most cases is a manifestation of a glucagon-secreting islet cell tumor of the pancreas (see p. 489).
The changes may resemble those seen in acrodermatitis enteropathica with psoriasiform hyperplasia, upper epidermal pallor, and overlying confluent parakeratosis. At other times there is focal or confluent necrosis of the upper epidermis with a preceding phase of pale, vacuolated keratinocytes. Subcorneal or intraepidermal clefting and pustulation may develop. Psoriasiform epidermal hyperplasia of any significant degree is present in only a minority of cases (Fig. 4.24).
Glucagonoma syndrome. There is very little vacuolation of keratinocytes, although it was present in another biopsy from this patient. (H & E)
The great imitator, syphilis, can sometimes present lesions, in the secondary phase, with a psoriasiform pattern (see p. 575).
It should be stressed that there is considerable variation in the histopathological appearances of secondary syphilis. Psoriasiform hyperplasia is more often seen in late lesions of secondary syphilis. A lichenoid tissue reaction may also be present and this combination of tissue reactions is very suggestive of syphilis, particularly if the infiltrate in the dermis forms in both the superficial and deep parts (Fig. 4.25). Plasma cells are commonly present, but they are not invariable.
Late secondary syphilis. The epidermal hyperplasia is less regular than is usual in psoriasiform hyperplasia. There are focal lichenoid changes. (H & E)
Introduction
1. Pinkus, H; Mehregan, AH, The primary histologic lesions of seborrheic dermatitis and psoriasis, J Invest Dermatol 46 (1966) 109–116.
2. Barr, RJ; Young Jr, EM, Psoriasiform and related papulosquamous disorders, J Cutan Pathol 12 (1985) 412–425.
Major psoriasiform dermatoses
3. Namazi, MR, Why is psoriasis uncommon in Africans? The influence of dietary factors on the expression of psoriasis, Int J Dermatol 43 (2004) 391–392.
4. Watson, W, Psoriasis: epidemiology and genetics, Dermatol Clin 2 (1984) 363–371.
5. Bell, LM; Sedlack, R; Beard, CM; et al., Incidence of psoriasis in Rochester, Minn, 1980–1983, Arch Dermatol 127 (1991) 1184–1187.
6. Christophers, E, Psoriasis – epidemiology and clinical spectrum, Clin Exp Dermatol 26 (2001) 314–320.
7. Bos, JD, Psoriasis, innate immunity, and gene pools, J Am Acad Dermatol 56 (2007) 468–471.
8. Huerta, C; Rivero, E; García Rodríguez, LA, Incidence and risk factors for psoriasis in the general population, Arch Dermatol 143 (2007) 1559–1565.
9. Ferrándiz, C; Bordas, X; García-Patos, V; et al., Prevalence of psoriasis in Spain (Epiderma Project: phase I), J Eur Acad Dermatol Venereol 15 (2001) 20–23.
10. Jullien, D; Barker, JN, Genetics of psoriasis, J Eur Acad Dermatol Venereol 20 (Suppl 2) (2006) 42–51.
11. Fry, L, Psoriasis, Br J Dermatol 119 (1988) 445–461.
12. Holubar, K; Fatović- Ferenčić, S, Papillary tip bleeding or the Auspitz phenomenon: A hero wrongly credited and a misnomer resolved, J Am Acad Dermatol 48 (2003) 263–264.
13. Gupta, MA; Gupta, AK; Kirkby, S; et al., Pruritus in psoriasis, Arch Dermatol 124 (1988) 1052–1057.
14. Nakamura, M; Toyoda, M; Morohashi, M, Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors, Br J Dermatol 149 (2003) 718–730.
15. Griffiths, CEM; Christophers, E; Barker, JNWN; et al., A classification of psoriasis vulgaris according to phenotype, Br J Dermatol 156 (2007) 258–262.
16. Van der Kerkhof, PCM, Clinical features, In: (Editors: Mier, PD; van der Kerkhof, PCM) Textbook of psoriasis (1986) Churchill Livingstone, Edinburgh, pp. 13–39.
17. Boisseau-Garsaud, A-M; Beylot-Barry, M; Doutre, M-S; et al., Psoriatic onycho-pachydermo-periostitis, Arch Dermatol 132 (1996) 176–180.